
\[\text{KMn}{{\text{O}}_{4}}\](acidic/alkaline) is not decolourised by:
A. Mohr salt
B. Oxalic acid
C. Benzene
D. Propene
Answer
594.9k+ views
Hint: Potassium permanganate consists of two ions i.e. potassium ion and permanganate ion that's why it is an ionic compound. Potassium permanganate comes in a category of the strongest oxidizing agents. Double bond and triple bonds on the side chain and not in the ring are necessary for the decolourisation of the potassium permanganate.
Complete step by step solution:
-We all know that benzene has a molecular formula of ${{\text{C}}_{6}}{{\text{H}}_{6}}$ and it consists of a double bond in a 6-membered ring structure.
-Also, benzene has a ∏-electron which undergoes the delocalisation process.
-So, it is not possible for benzene to decolourise the \[\text{KMn}{{\text{O}}_{4}}\] .
-Hence, option C. is the correct answer.
-Mohr salt or commonly called Ferrous Ammonium Sulphate consists of a double bond which has the ability to decolourise the potassium permanganate.
-The chemical reaction is:
$\text{2KMn}{{\text{O}}_{4}}\text{ + 10FeS}{{\text{O}}_{4}}\text{ + 8}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\text{ }{{\text{K}}_{2}}\text{S}{{\text{O}}_{4}}\text{ + 2MnS}{{\text{O}}_{4}}\text{ + 5F}{{\text{e}}_{2}}{{\left( \text{S}{{\text{O}}_{4}} \right)}_{3}}\text{ + 8}{{\text{H}}_{2}}\text{O}$
-So, option A. is not the correct answer.
-Similarly, oxalic acid can also react with the potassium permanganate and decolourise the solution from pink to colourless.
-The chemical reaction is:
$\text{2KMn}{{\text{O}}_{4}}\text{ + 5}{{\text{C}}_{2}}{{\text{H}}_{2}}{{\text{O}}_{4}}\text{ + 3}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$→ ${{\text{K}}_{2}}\text{S}{{\text{O}}_{4}}\text{ + 2MnS}{{\text{O}}_{4}}\text{ + 10C}{{\text{O}}_{2}}\text{ + 8}{{\text{H}}_{2}}\text{O}$
-So, option B. is also an incorrect answer.
-In propene, there is a presence of a double bond as we can see in the below-given structure:
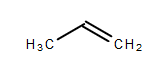
-So, due to the presence of a double bond in propene, it can decolourise the potassium permanganate.
So, the correct answer is “Option C”.
Note: Potassium permanganate can be used to test the presence of unsaturated compounds because potassium permanganate does not react with the alkanes and hence, no change in the colour takes place.
Complete step by step solution:
-We all know that benzene has a molecular formula of ${{\text{C}}_{6}}{{\text{H}}_{6}}$ and it consists of a double bond in a 6-membered ring structure.
-Also, benzene has a ∏-electron which undergoes the delocalisation process.
-So, it is not possible for benzene to decolourise the \[\text{KMn}{{\text{O}}_{4}}\] .
-Hence, option C. is the correct answer.
-Mohr salt or commonly called Ferrous Ammonium Sulphate consists of a double bond which has the ability to decolourise the potassium permanganate.
-The chemical reaction is:
$\text{2KMn}{{\text{O}}_{4}}\text{ + 10FeS}{{\text{O}}_{4}}\text{ + 8}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\text{ }{{\text{K}}_{2}}\text{S}{{\text{O}}_{4}}\text{ + 2MnS}{{\text{O}}_{4}}\text{ + 5F}{{\text{e}}_{2}}{{\left( \text{S}{{\text{O}}_{4}} \right)}_{3}}\text{ + 8}{{\text{H}}_{2}}\text{O}$
-So, option A. is not the correct answer.
-Similarly, oxalic acid can also react with the potassium permanganate and decolourise the solution from pink to colourless.
-The chemical reaction is:
$\text{2KMn}{{\text{O}}_{4}}\text{ + 5}{{\text{C}}_{2}}{{\text{H}}_{2}}{{\text{O}}_{4}}\text{ + 3}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$→ ${{\text{K}}_{2}}\text{S}{{\text{O}}_{4}}\text{ + 2MnS}{{\text{O}}_{4}}\text{ + 10C}{{\text{O}}_{2}}\text{ + 8}{{\text{H}}_{2}}\text{O}$
-So, option B. is also an incorrect answer.
-In propene, there is a presence of a double bond as we can see in the below-given structure:

-So, due to the presence of a double bond in propene, it can decolourise the potassium permanganate.
So, the correct answer is “Option C”.
Note: Potassium permanganate can be used to test the presence of unsaturated compounds because potassium permanganate does not react with the alkanes and hence, no change in the colour takes place.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




