
Label the given diagram showing lead storage battery.
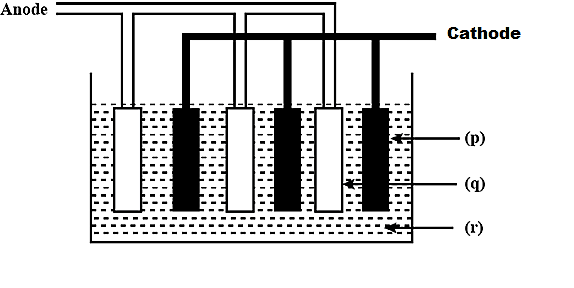
(A) \[{\rm{p - Pb, q - Pb}}{{\rm{O}}_2},{\rm{r - 5M }}{{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4}\]
(B) \[{\rm{p - Pb}}{{\rm{O}}_{\rm{2}}}{\rm{,q - Pb,r - conc}}{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]
(C) \[{\rm{p - Pb, q - Pb}}{{\rm{O}}_2},{\rm{r - 50\% }}{{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4}\]
(D) \[{\rm{p - Pb}}{{\rm{O}}_{\rm{2}}}{\rm{,q - Pb,r - dil}}{\rm{. 38\% }}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]

Answer
564.9k+ views
Hint:. Lead storage batteries are also known as the lead acid battery. It is a kind of rechargeable battery. Lead storage batteries are the energy storage device. It is a secondary storage battery. This secondary storage battery can undergo reversible chemical reaction.
Complete step by step answer:
- Lead storage battery is also known as the secondary storage battery. In the lead storage battery, the electrical energy will be converted into chemical energy by the application of certain external sources. This is known as charging.
- Similarly, the chemical energy will be converted into electrical energy whenever it is required. This is called discharging.
- In the lead storage battery, both charging and discharging can take place.
- During the discharging process, both positive and the negative plate will be converted into \[{\rm{PbS}}{{\rm{O}}_4}\]. The electrolyte solution, i.e. dissolved \[{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]will be lost and it will become water.
- The electron conduction can take place from the negative plate to the positive plate.
- The chemical reaction taking place during the discharging process is given below:
The reaction in the negative plate is
\[{\rm{Pb(s) + HSO}}_{\rm{4}}^{\rm{ - }}{\rm{(aq)}} \to {\rm{PbS}}{{\rm{O}}_{\rm{4}}}{\rm{(s) + }}{{\rm{H}}^{\rm{ + }}}{\rm{(aq) + 2}}{{\rm{e}}^{\rm{ - }}}\]
The reaction in the positive plate is :
\[{\rm{Pb}}{{\rm{O}}_2}{\rm{(s) + HSO}}_{\rm{4}}^{\rm{ - }}{\rm{(aq) + 3}}{{\rm{H}}^{\rm{ + }}}{\rm{ + 2}}{{\rm{e}}^{\rm{ - }}} \to {\rm{PbS}}{{\rm{O}}_{\rm{4}}}{\rm{(s) + }}{{\rm{H}}_{\rm{2}}}{\rm{O(l)}}\]
The overall reaction is :
\[{\rm{Pb(s) + Pb}}{{\rm{O}}_{\rm{2}}}{\rm{(s) + 2}}{{\rm{H}}^{\rm{ + }}}{\rm{ + 2HSO}}_{\rm{4}}^{\rm{ - }} \to {\rm{PbS}}{{\rm{O}}_{\rm{4}}}{\rm{(s) + }}{{\rm{H}}_{\rm{2}}}{\rm{O(l)}}\]
The charging process is reverse of discharging process.
Hence,
\[{\rm{p - Pb}}{{\rm{O}}_{\rm{2}}}\],
\[{\rm{q - Pb}}\],
\[{\rm{r - dil}}{\rm{. 38\% }}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]
Therefore, the correct answer is option (D) \[{\rm{p - Pb}}{{\rm{O}}_{\rm{2}}}{\rm{,q - Pb,r - dil}}{\rm{. 38\% }}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]
Additional information: Comparison between Primary cell and Secondary cell:
Note: Lead storage batteries are composed of very harmful chemicals. If it is not maintained properly, it can be hazardous to our health. The sulphuric acid used in the battery is highly corrosive in nature. Therefore, after the end life of the battery, it has to be disposed properly.
Complete step by step answer:
- Lead storage battery is also known as the secondary storage battery. In the lead storage battery, the electrical energy will be converted into chemical energy by the application of certain external sources. This is known as charging.
- Similarly, the chemical energy will be converted into electrical energy whenever it is required. This is called discharging.
- In the lead storage battery, both charging and discharging can take place.
- During the discharging process, both positive and the negative plate will be converted into \[{\rm{PbS}}{{\rm{O}}_4}\]. The electrolyte solution, i.e. dissolved \[{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]will be lost and it will become water.
- The electron conduction can take place from the negative plate to the positive plate.
- The chemical reaction taking place during the discharging process is given below:
The reaction in the negative plate is
\[{\rm{Pb(s) + HSO}}_{\rm{4}}^{\rm{ - }}{\rm{(aq)}} \to {\rm{PbS}}{{\rm{O}}_{\rm{4}}}{\rm{(s) + }}{{\rm{H}}^{\rm{ + }}}{\rm{(aq) + 2}}{{\rm{e}}^{\rm{ - }}}\]
The reaction in the positive plate is :
\[{\rm{Pb}}{{\rm{O}}_2}{\rm{(s) + HSO}}_{\rm{4}}^{\rm{ - }}{\rm{(aq) + 3}}{{\rm{H}}^{\rm{ + }}}{\rm{ + 2}}{{\rm{e}}^{\rm{ - }}} \to {\rm{PbS}}{{\rm{O}}_{\rm{4}}}{\rm{(s) + }}{{\rm{H}}_{\rm{2}}}{\rm{O(l)}}\]
The overall reaction is :
\[{\rm{Pb(s) + Pb}}{{\rm{O}}_{\rm{2}}}{\rm{(s) + 2}}{{\rm{H}}^{\rm{ + }}}{\rm{ + 2HSO}}_{\rm{4}}^{\rm{ - }} \to {\rm{PbS}}{{\rm{O}}_{\rm{4}}}{\rm{(s) + }}{{\rm{H}}_{\rm{2}}}{\rm{O(l)}}\]
The charging process is reverse of discharging process.
Hence,
\[{\rm{p - Pb}}{{\rm{O}}_{\rm{2}}}\],
\[{\rm{q - Pb}}\],
\[{\rm{r - dil}}{\rm{. 38\% }}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]
Therefore, the correct answer is option (D) \[{\rm{p - Pb}}{{\rm{O}}_{\rm{2}}}{\rm{,q - Pb,r - dil}}{\rm{. 38\% }}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]
Additional information: Comparison between Primary cell and Secondary cell:
| PRIMARY CELL | SECONDARY CELL |
| It has high density and is very easy to use. | It has a very low density |
| Primary cell is not rechargeable. | Secondary cell is rechargeable |
| It is very Cheap | It is costly. |
| It has high internal resistance | It has low internal resistance. |
| It undergoes irreversible chemical reaction | It undergoes reversible chemical reaction |
Note: Lead storage batteries are composed of very harmful chemicals. If it is not maintained properly, it can be hazardous to our health. The sulphuric acid used in the battery is highly corrosive in nature. Therefore, after the end life of the battery, it has to be disposed properly.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




