
Lactic acid and glycolic acid are the monomers used for preparation of polymer:
A. Nylon-2-nylon-6
B. Dextron
C. PHBV
D. Buna-N
Answer
512.1k+ views
Hint :Polymers are materials made up of continuous chains of molecules that repeat themselves. Depending on the sort of molecules bound and how they are linked, the materials have distinct properties. Rubber and polyester are two materials that flex and stretch. Plastics, which are man made polymers, are frequently referred to as polymers.
Complete Step By Step Answer:
Glycolic acid is a monomer of the thermoplastic polymer polyglycolic acid. In addition, lactic acid is the monomer of polylactic acid, a thermoplastic polymer. A copolymer is formed when these two monomers react.
The monomers utilised to make Dextron are lactic acid and glycolic acid. Dextron is an ester-linked copolymer.
Polyester copolymer Dextron is a form of polyester copolymer. Polyester is made through condensation polymerization, which involves the reaction of carboxylic acid functional groups with hydroxyl groups to remove water molecules.
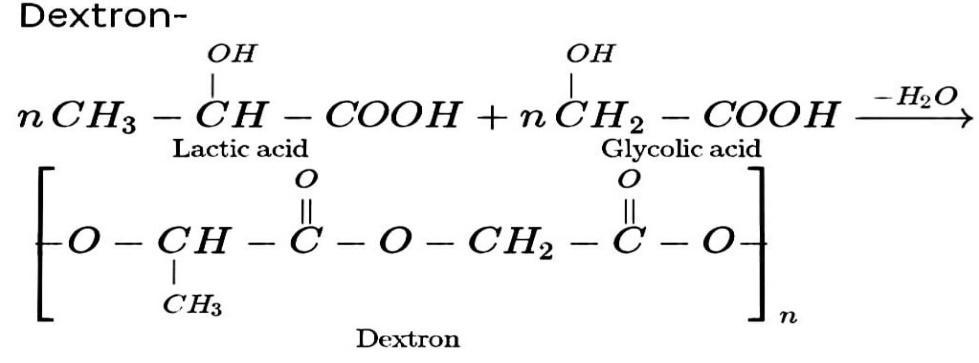
Hence, lactic acid and glycolic acid are the monomers used for preparation of dextron
So, the correct option is: B. Dextron
Additional information:
It works as a friction reducer. It's used in lubricants to minimise friction on the surface. To reduce friction and wear in machine components, friction modifiers are added to lubricants. They're especially useful in the boundary lubrication regime, where they can prevent solid surfaces from making direct contact, reducing friction and wear significantly.
Note :
Make sure you don't mix up Dextron, Dextrin, and Dextran. Dextran is a type of carbohydrate and a polysaccharide generated from glucose. Remember that two hydroxyl groups cannot combine to remove water molecules in this condensation reaction.
Complete Step By Step Answer:
Glycolic acid is a monomer of the thermoplastic polymer polyglycolic acid. In addition, lactic acid is the monomer of polylactic acid, a thermoplastic polymer. A copolymer is formed when these two monomers react.
The monomers utilised to make Dextron are lactic acid and glycolic acid. Dextron is an ester-linked copolymer.
Polyester copolymer Dextron is a form of polyester copolymer. Polyester is made through condensation polymerization, which involves the reaction of carboxylic acid functional groups with hydroxyl groups to remove water molecules.

Hence, lactic acid and glycolic acid are the monomers used for preparation of dextron
So, the correct option is: B. Dextron
Additional information:
It works as a friction reducer. It's used in lubricants to minimise friction on the surface. To reduce friction and wear in machine components, friction modifiers are added to lubricants. They're especially useful in the boundary lubrication regime, where they can prevent solid surfaces from making direct contact, reducing friction and wear significantly.
Note :
Make sure you don't mix up Dextron, Dextrin, and Dextran. Dextran is a type of carbohydrate and a polysaccharide generated from glucose. Remember that two hydroxyl groups cannot combine to remove water molecules in this condensation reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




