
Lateral overlap of p-orbitals leads to the formation of ______.
A) ${{\pi }}$-bond
B) Metallic bond
C) Sigma bond
D) Ionic bond
Answer
569.4k+ views
Hint:We know that the bonds are formed by the overlapping of orbitals. The extent of orbital overlapping depends on the size of the two participating atoms, and the valence electrons. The concentration of orbitals on the adjacent atoms in the same region is known as orbital overlapping.
Complete answer :
We know that the bonds are formed by the overlapping of orbitals. The extent of orbital overlapping depends on the size of the two participating atoms, and the valence electrons. The concentration of orbitals on the adjacent atoms in the same region is known as orbital overlapping.
Orbital overlapping leads to the formation of two types of bonds:
i) Sigma bond
ii) pi-bond
The covalent bonds formed by the axial overlap of s-orbitals are known as $\sigma $-bonds (sigma bond). The covalent bonds formed by the lateral overlap of p-orbitals or the half-filled atomic orbitals are known as ${{\pi }}$-bonds (pi bonds).
The formation of ${{\pi }}$-bond occurs as follows:
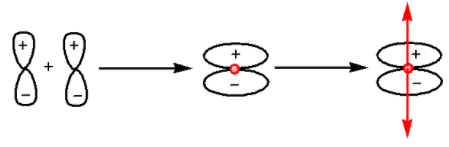
From the diagram we can see that ${{\pi }}$-bond is unsymmetrical.Thus, lateral overlap of p-orbitals leads to the formation of ${{\pi }}$-bond.
Therefore, the correct answer is option (A) ${{\pi }}$-bond.
Note:The extent of overlapping in ${{\pi }}$-bond is very less and thus, ${{\pi }}$-bond is weak in nature. In ${{\pi }}$-bond, the electron density is concentrated in the region which is perpendicular to the axis of the bond. A double covalent bond exists when one ${{\pi }}$-bond and one $\sigma $-bond is formed. A triple covalent bond exists when two ${{\pi }}$-bond and one $\sigma $-bond is formed.
Complete answer :
We know that the bonds are formed by the overlapping of orbitals. The extent of orbital overlapping depends on the size of the two participating atoms, and the valence electrons. The concentration of orbitals on the adjacent atoms in the same region is known as orbital overlapping.
Orbital overlapping leads to the formation of two types of bonds:
i) Sigma bond
ii) pi-bond
The covalent bonds formed by the axial overlap of s-orbitals are known as $\sigma $-bonds (sigma bond). The covalent bonds formed by the lateral overlap of p-orbitals or the half-filled atomic orbitals are known as ${{\pi }}$-bonds (pi bonds).
The formation of ${{\pi }}$-bond occurs as follows:

From the diagram we can see that ${{\pi }}$-bond is unsymmetrical.Thus, lateral overlap of p-orbitals leads to the formation of ${{\pi }}$-bond.
Therefore, the correct answer is option (A) ${{\pi }}$-bond.
Note:The extent of overlapping in ${{\pi }}$-bond is very less and thus, ${{\pi }}$-bond is weak in nature. In ${{\pi }}$-bond, the electron density is concentrated in the region which is perpendicular to the axis of the bond. A double covalent bond exists when one ${{\pi }}$-bond and one $\sigma $-bond is formed. A triple covalent bond exists when two ${{\pi }}$-bond and one $\sigma $-bond is formed.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




