
What is the lattice enthalpy of an ionic crystal? Give the relation.
Answer
589.2k+ views
Hint: In an ionic crystal, we have anions as well as cations and they form the crystal lattice structure. To form or dissociate this lattice there is either loss of gain of energy. Change in energy is the enthalpy of any system.
Complete step by step answer:
We know that in a crystalline solid, when the ions are combined, it causes energy release. We know this energy by the name lattice energy, the change in energy in this process if basically the lattice enthalpy.
The term lattice enthalpy if used for both i.e. the change in energy when 1 mole of any substance is formed from their corresponding ionic forms or we can also say that it is the energy required for separating 1 mole of a solid crystal into its corresponding ions.
However, to be more appropriate about the energy required for breaking of the crystal lattice and the formation of the crystal lattice, we use the terms lattice dissociation enthalpy and lattice formation enthalpy respectively. We can also deduce that it is the force acting between the ions in a crystal lattice. Higher the lattice energy, stronger is the force between them.
We know that the formation of a crystal lattice is always exothermic and thus the value of $\Delta {{H}_{lattice}}$ is also negative. Lattice dissociation enthalpy is always positive and lattice formation enthalpy is always negative. To calculate lattice energy, we use Born-Haber’s cycle. Let us take sodium chloride and try to find its lattice energy.
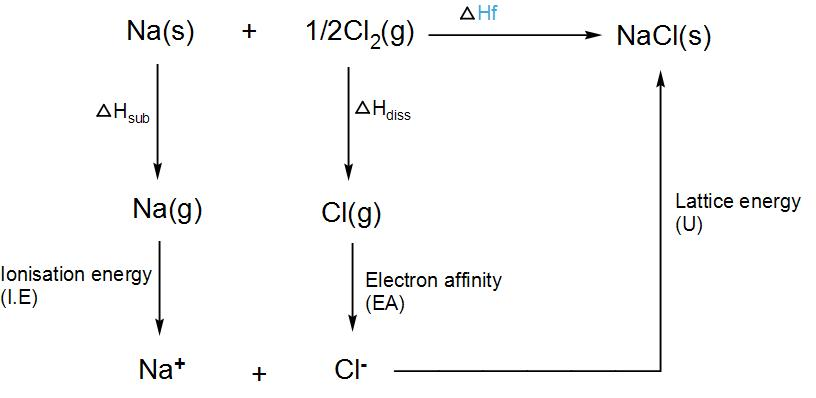
Thus, the lattice enthalpy of sodium chloride will be- $\Delta H_{f}^{\circ }=\Delta {{H}_{sub}}+I.E+\Delta {{H}_{diss.}}+U$
Note: There are two factors that affect the lattice energy of any substance. Firstly it is the charge on the ions and second is the radius of the ions. Higher is the charge on each ion, higher will be the lattice energy. Lower the internuclear distances among the ions in an ionic crystal, higher will be their lattice energy i.e. closer the two ions are, higher is the force of attraction between them.
Complete step by step answer:
We know that in a crystalline solid, when the ions are combined, it causes energy release. We know this energy by the name lattice energy, the change in energy in this process if basically the lattice enthalpy.
The term lattice enthalpy if used for both i.e. the change in energy when 1 mole of any substance is formed from their corresponding ionic forms or we can also say that it is the energy required for separating 1 mole of a solid crystal into its corresponding ions.
However, to be more appropriate about the energy required for breaking of the crystal lattice and the formation of the crystal lattice, we use the terms lattice dissociation enthalpy and lattice formation enthalpy respectively. We can also deduce that it is the force acting between the ions in a crystal lattice. Higher the lattice energy, stronger is the force between them.
We know that the formation of a crystal lattice is always exothermic and thus the value of $\Delta {{H}_{lattice}}$ is also negative. Lattice dissociation enthalpy is always positive and lattice formation enthalpy is always negative. To calculate lattice energy, we use Born-Haber’s cycle. Let us take sodium chloride and try to find its lattice energy.

Thus, the lattice enthalpy of sodium chloride will be- $\Delta H_{f}^{\circ }=\Delta {{H}_{sub}}+I.E+\Delta {{H}_{diss.}}+U$
Note: There are two factors that affect the lattice energy of any substance. Firstly it is the charge on the ions and second is the radius of the ions. Higher is the charge on each ion, higher will be the lattice energy. Lower the internuclear distances among the ions in an ionic crystal, higher will be their lattice energy i.e. closer the two ions are, higher is the force of attraction between them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




