
What is the lewis dot structure of nitrate ion?
Answer
530.4k+ views
Hint : The lewis dot structure includes marking the valence electrons of every element included in the compound with the electrons used in the covalent as well as ionic bonds. For simplicity, we can use different symbols to show electrons of different elements.
Complete Step By Step Answer:
Lewis structures or Lewis dot structures show the bonding between atoms and molecules, as well as the lone pair of electrons from the valence orbit, if any present.
Here, we are asked about the Lewis structure for Nitrate ion. We must get the formula for Nitrate ion.
Nitrate ion is formed from Hydrogen Nitrate, when the hydrogen atom leaves the compound as a positive ion, leaving its electron behind, which makes the charge on nitrate ion negative.
Hence, the Chemical formula for nitrate ion is $ N{{O}_{3}}^{-} $
Now, let us write the Lewis structure for the nitrate ion.
First, we need to calculate the total number of valence electrons of oxygen and nitrogen including the negative charge.
We know there are five valence electrons in nitrogen and six valence electrons in oxygen.
Hence, the total number of electrons is = $ 5+6+6+6+1 $ = $ \;24 $
Now, we need to understand the bonding and the lone pairs of the atoms
In nitrate ion, one of the oxygen shares two of its valence electrons, and forms a double covalent bond with nitrogen. Hence, out of six valence electrons two are used in bonding and the rest four are shown as two lone pairs.
One of the oxygen, due to higher electronegativity, makes a coordinate covalent bond in which both the electrons belong to nitrogen. Hence, none of its valence electrons are used in bonding and hence there are three lone pairs of electrons.
The last oxygen forms a single covalent bond with nitrogen by sharing one electron. It is also combined with hydrogen by an ionic bond, which in this case was removed as a positive ion. Hence, out of six valence electrons, four are shown as two lone pairs, one is shown as a covalent bond with nitrogen and one is paired with an electron from hydrogen.
Hence, from the above results, the Lewis structure can be shown as below,
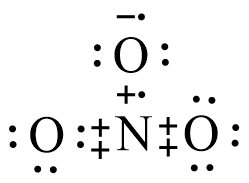
Here, plus sign shows electrons of nitrogen, dot shows electrons of oxygen, and minus sign shows electron of hydrogen.
The Lewis dot structure can also be shown with bonds and lone pairs as shown below,

Note :
The fact to remember here is that the nitrate ion is formed from Hydrogen Nitrate and hence, will always have a negative charge due to higher electronegativity. The negative or positive should always be included in the total counting of electrons of the compound. Here, the factors like bond length and bond angle can be ignored safely. Hence, the structure is not considered wrong if the bond angle is wrong. The important thing is to show the correct sharing of electrons.
Complete Step By Step Answer:
Lewis structures or Lewis dot structures show the bonding between atoms and molecules, as well as the lone pair of electrons from the valence orbit, if any present.
Here, we are asked about the Lewis structure for Nitrate ion. We must get the formula for Nitrate ion.
Nitrate ion is formed from Hydrogen Nitrate, when the hydrogen atom leaves the compound as a positive ion, leaving its electron behind, which makes the charge on nitrate ion negative.
Hence, the Chemical formula for nitrate ion is $ N{{O}_{3}}^{-} $
Now, let us write the Lewis structure for the nitrate ion.
First, we need to calculate the total number of valence electrons of oxygen and nitrogen including the negative charge.
We know there are five valence electrons in nitrogen and six valence electrons in oxygen.
Hence, the total number of electrons is = $ 5+6+6+6+1 $ = $ \;24 $
Now, we need to understand the bonding and the lone pairs of the atoms
In nitrate ion, one of the oxygen shares two of its valence electrons, and forms a double covalent bond with nitrogen. Hence, out of six valence electrons two are used in bonding and the rest four are shown as two lone pairs.
One of the oxygen, due to higher electronegativity, makes a coordinate covalent bond in which both the electrons belong to nitrogen. Hence, none of its valence electrons are used in bonding and hence there are three lone pairs of electrons.
The last oxygen forms a single covalent bond with nitrogen by sharing one electron. It is also combined with hydrogen by an ionic bond, which in this case was removed as a positive ion. Hence, out of six valence electrons, four are shown as two lone pairs, one is shown as a covalent bond with nitrogen and one is paired with an electron from hydrogen.
Hence, from the above results, the Lewis structure can be shown as below,
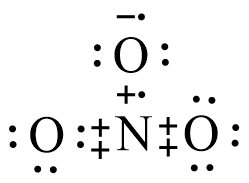
Here, plus sign shows electrons of nitrogen, dot shows electrons of oxygen, and minus sign shows electron of hydrogen.
The Lewis dot structure can also be shown with bonds and lone pairs as shown below,

Note :
The fact to remember here is that the nitrate ion is formed from Hydrogen Nitrate and hence, will always have a negative charge due to higher electronegativity. The negative or positive should always be included in the total counting of electrons of the compound. Here, the factors like bond length and bond angle can be ignored safely. Hence, the structure is not considered wrong if the bond angle is wrong. The important thing is to show the correct sharing of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




