
What is the Lewis structure of ozone (\[{O_3}\])?
Answer
504.9k+ views
Hint: We know that ozone prevents the atmosphere from ultraviolet rays from sunlight. Oxygen is used for respiration in human beings. There are two types of forces between the molecules. There are intermolecular forces and intramolecular forces. The oxygen molecules have intramolecular forces in the atmosphere. But, ozone molecules have intermolecular forces in the atmosphere. Ozone is present in between the layers of atmosphere and stratosphere. The ozone comprises the higher concentration in the atmosphere. The ozone blanket comprises the high concentration in the stratosphere. The range of the ozone blanket in the stratosphere is around \[18 - 26km\].
Complete answer:
The Lewis structure is nothing but the structural representation of molecules.
The structural representation of ozone (\[{O_3}\]) by Lewis structure is given below,
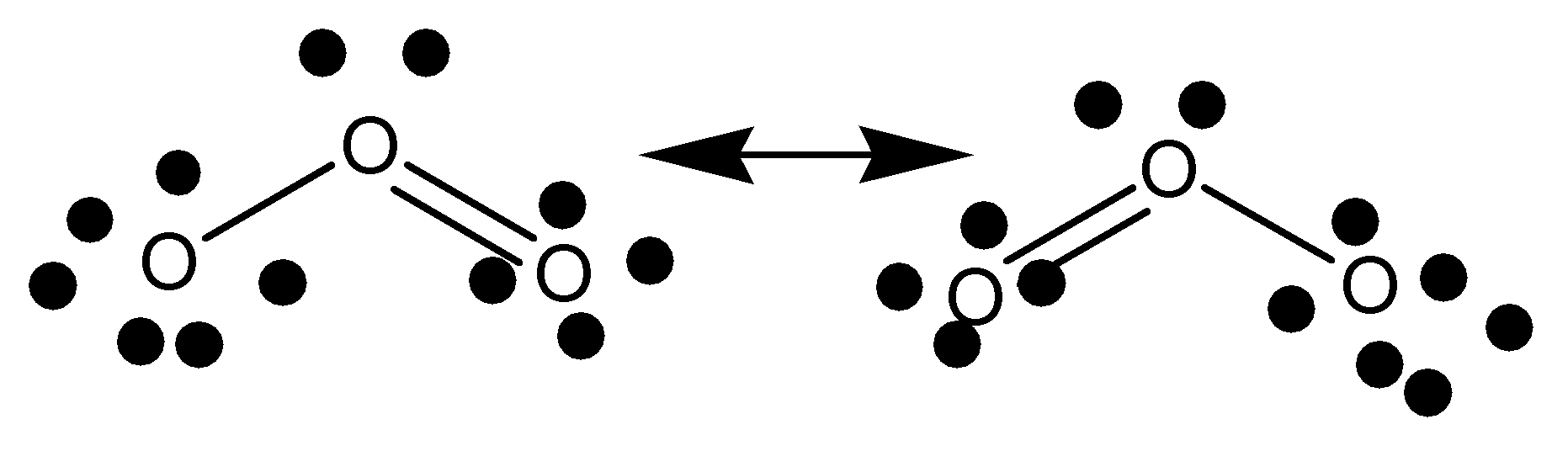
Note:
We need to know that oxygen is one of the element periodic tables. The symbol of oxygen is \[{\text{O}}\]. The atomic number of oxygen is \[8\]. The symbol of the oxygen molecule is \[{{\text{O}}_{\text{2}}}\] and it is a diatomic molecule, the number of atoms in the molecule is two. The symbol of the ozone molecule is \[{{\text{O}}_{\text{3}}}\] and it is a triatomic molecule, the number of atoms in the molecule is three. At standard pressure, the boiling point of ozone is greater than oxygen. The boiling point of difference in oxygen and ozone at standard pressure, because of the volatile nature of oxygen in the atmosphere. But this kind of volatility is absent in ozone. Ozone molecules are heavier than oxygen atoms. Heaviness of the ozone molecule is due to the electron cloud. In the atmosphere, consequently greater dispersive forces in ozone increases the intermolecular forces.
Complete answer:
The Lewis structure is nothing but the structural representation of molecules.
The structural representation of ozone (\[{O_3}\]) by Lewis structure is given below,

Note:
We need to know that oxygen is one of the element periodic tables. The symbol of oxygen is \[{\text{O}}\]. The atomic number of oxygen is \[8\]. The symbol of the oxygen molecule is \[{{\text{O}}_{\text{2}}}\] and it is a diatomic molecule, the number of atoms in the molecule is two. The symbol of the ozone molecule is \[{{\text{O}}_{\text{3}}}\] and it is a triatomic molecule, the number of atoms in the molecule is three. At standard pressure, the boiling point of ozone is greater than oxygen. The boiling point of difference in oxygen and ozone at standard pressure, because of the volatile nature of oxygen in the atmosphere. But this kind of volatility is absent in ozone. Ozone molecules are heavier than oxygen atoms. Heaviness of the ozone molecule is due to the electron cloud. In the atmosphere, consequently greater dispersive forces in ozone increases the intermolecular forces.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




