
What is light emitting diode (LED)? Explain its working principle and state factor on which the colour of light emitted by it depends.
Answer
520.3k+ views
Hint: Recombination of the charge carrier occurs in the LED. When the coloured light is emitted at a specified wavelength of spectral when the LED is forward biased. The wavelength of the light emitted has its colour depending on the energy gap.
Complete step by step answer:
A light emitting diode (LED) is a forward biased p-n junction diode That emits visible light when energized. LED work on the phenomenon of electroluminescence. The colour of light emitted depends on the semiconducting material used.
When light emitting diodes are forward biased, the electrons from the n-type material cross the p-n junction and recombine with holes in the p-type material. These free electrons are in the conduction band and at a higher energy level than the holes in the valence band. The recombination of electrons releases energy in the form of heat and light. In Germanium and Silicon diodes, almost the entire energy is given up in the form of heat and emitted light is in IR region. But, in materials like Arsenide, Gallium Phosphate the number of photons of light energy is emitted which is sufficient to produce intense visible light.
The symbol of LED is as shown in fig.
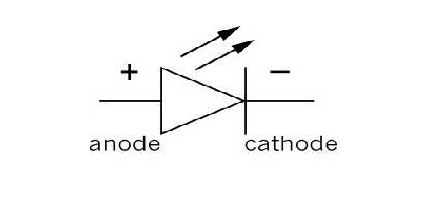
The V-I characteristics of a LED is similar to that of a Si or Ge junction diode. But the threshold voltages are much higher. The reverse breakdown voltage of LED is very low (about 5V).
The wavelength of the light emitted, and therefore its colour, depends on the energy gap of the materials forming the p-n junction. A normal diode, typically made of SI or Ge, emits invisible far IR radiation. But the materials used for an LED have band gap energies corresponding to visible light.
Wavelength of light emitted,$\lambda = \dfrac{{hc}}{{{E_g}}}$
Where, ${E_g}$ is the energy gap of the materials forming the p-n junction, h is Planck’s constant, c is the speed of light.
Visible LEDs are available for variety colours. Energy range of the visible region is 1.8eV to 2.8eV. hence, those materials with energy gaps in this range can be used. Si and Ge cannot be used in LED, because their energy gaps are less than 1.8eV. Gallium arsenide-phosphide is used for making LEDs of different colours.
Gallium phosphide $ \to {\text{ red or green, Gallium arsenide phosphide}} \to {\text{red or yellow}}$
${\text{Gallium arsenide}} \to {\text{IR or red, Gallium nitride}} \to {\text{blue}}$
Note:
LCD stands for liquid crystal display. LCDs primarily use fluorescent lights that are usually placed behind the screens. All LCDs are not the subset of the LEDs. LCDs are usually thicker and lack energy when compared to LEDs .
Complete step by step answer:
A light emitting diode (LED) is a forward biased p-n junction diode That emits visible light when energized. LED work on the phenomenon of electroluminescence. The colour of light emitted depends on the semiconducting material used.
When light emitting diodes are forward biased, the electrons from the n-type material cross the p-n junction and recombine with holes in the p-type material. These free electrons are in the conduction band and at a higher energy level than the holes in the valence band. The recombination of electrons releases energy in the form of heat and light. In Germanium and Silicon diodes, almost the entire energy is given up in the form of heat and emitted light is in IR region. But, in materials like Arsenide, Gallium Phosphate the number of photons of light energy is emitted which is sufficient to produce intense visible light.
The symbol of LED is as shown in fig.

The V-I characteristics of a LED is similar to that of a Si or Ge junction diode. But the threshold voltages are much higher. The reverse breakdown voltage of LED is very low (about 5V).
The wavelength of the light emitted, and therefore its colour, depends on the energy gap of the materials forming the p-n junction. A normal diode, typically made of SI or Ge, emits invisible far IR radiation. But the materials used for an LED have band gap energies corresponding to visible light.
Wavelength of light emitted,$\lambda = \dfrac{{hc}}{{{E_g}}}$
Where, ${E_g}$ is the energy gap of the materials forming the p-n junction, h is Planck’s constant, c is the speed of light.
Visible LEDs are available for variety colours. Energy range of the visible region is 1.8eV to 2.8eV. hence, those materials with energy gaps in this range can be used. Si and Ge cannot be used in LED, because their energy gaps are less than 1.8eV. Gallium arsenide-phosphide is used for making LEDs of different colours.
Gallium phosphide $ \to {\text{ red or green, Gallium arsenide phosphide}} \to {\text{red or yellow}}$
${\text{Gallium arsenide}} \to {\text{IR or red, Gallium nitride}} \to {\text{blue}}$
Note:
LCD stands for liquid crystal display. LCDs primarily use fluorescent lights that are usually placed behind the screens. All LCDs are not the subset of the LEDs. LCDs are usually thicker and lack energy when compared to LEDs .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




