
List out some strong and weak electrolytes.
Answer
508.5k+ views
Hint: A chemical compound which dissociates into its respective ions on dissolving it in a solvent (generally water) is known as an electrolyte. The ions formed in the solution are positively charged i.e., cations and negatively charged ions i.e., anions which are free to move and thus, conducts electricity in the solution.
Complete answer:
A strong electrolyte is a molecule which completely dissociates in a solution. These ions are the good conductors of electricity in the aqueous solution. A concentrated solution which consists of strong electrolytes has a lower vapour pressure as compared to pure water at the same temperature. Strong electrolytes include strong acids, strong bases and soluble ionic salts.
Examples of strong electrolyte are as follows:
1. Hydrochloric acid $(HCl)$
2. Sodium hydroxide$(NaOH)$
3. Sodium chloride $(NaCl)$
A weak electrolyte is a molecule which does not completely dissociate in a solution i.e., the molecule only breaks partially into ions in the solvent. These are the weak conductors of electric current. Weak electrolytes include weak acids, weak bases and compounds containing nitrogen compounds.
Examples of weak electrolyte are as follows:
1. Hydrofluoric acid $(HF)$
2. Acetic acid $(C{H_3}COOH)$
3. Ammonia $(N{H_3})$
Additional information-
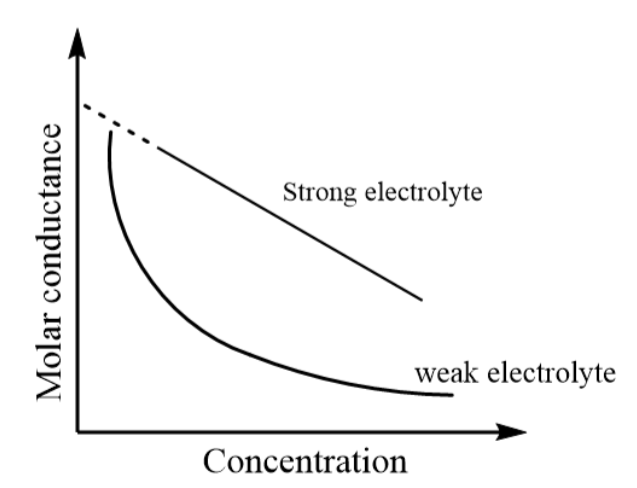
As the strong electrolytes completely dissociate in the solvent, so the number of ions in the solution remain constant and at higher concentrations, there exist a greater inter ionic attractive forces due to which retardation in the motion of ions is observed. Thus, on increasing the concentration of strong electrolytes, the molar conductance decreases linearly.
Conversely, the weak electrolytes consist of a lower degree of dissociation that means the ratio of ions is not fixed at the given temperature of the solvent. Thus, with an increase in concentration of weak electrolytes, a steep decrease is observed in the molar conductivity of the solution.
The graph of molar conductance versus concentration for weak and strong electrolytes is represented as follows:
Note:
It is important to note that nonelectrolytes are the chemical compounds which do not dissociate at all in an aqueous solution and thus, do not conduct electricity. The major difference between electrolytes and nonelectrolytes is that the electrolytes are composed of ionic bonds whereas the non-electrolytes are composed of the covalent bonds.
Complete answer:
A strong electrolyte is a molecule which completely dissociates in a solution. These ions are the good conductors of electricity in the aqueous solution. A concentrated solution which consists of strong electrolytes has a lower vapour pressure as compared to pure water at the same temperature. Strong electrolytes include strong acids, strong bases and soluble ionic salts.
Examples of strong electrolyte are as follows:
1. Hydrochloric acid $(HCl)$
2. Sodium hydroxide$(NaOH)$
3. Sodium chloride $(NaCl)$
A weak electrolyte is a molecule which does not completely dissociate in a solution i.e., the molecule only breaks partially into ions in the solvent. These are the weak conductors of electric current. Weak electrolytes include weak acids, weak bases and compounds containing nitrogen compounds.
Examples of weak electrolyte are as follows:
1. Hydrofluoric acid $(HF)$
2. Acetic acid $(C{H_3}COOH)$
3. Ammonia $(N{H_3})$
Additional information-
As the strong electrolytes completely dissociate in the solvent, so the number of ions in the solution remain constant and at higher concentrations, there exist a greater inter ionic attractive forces due to which retardation in the motion of ions is observed. Thus, on increasing the concentration of strong electrolytes, the molar conductance decreases linearly.
Conversely, the weak electrolytes consist of a lower degree of dissociation that means the ratio of ions is not fixed at the given temperature of the solvent. Thus, with an increase in concentration of weak electrolytes, a steep decrease is observed in the molar conductivity of the solution.
The graph of molar conductance versus concentration for weak and strong electrolytes is represented as follows:

Note:
It is important to note that nonelectrolytes are the chemical compounds which do not dissociate at all in an aqueous solution and thus, do not conduct electricity. The major difference between electrolytes and nonelectrolytes is that the electrolytes are composed of ionic bonds whereas the non-electrolytes are composed of the covalent bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




