
When m- chloro-benzaldehyde is treated with a 50% $KOH$ solution, the product(s) obtained is (are):
A.
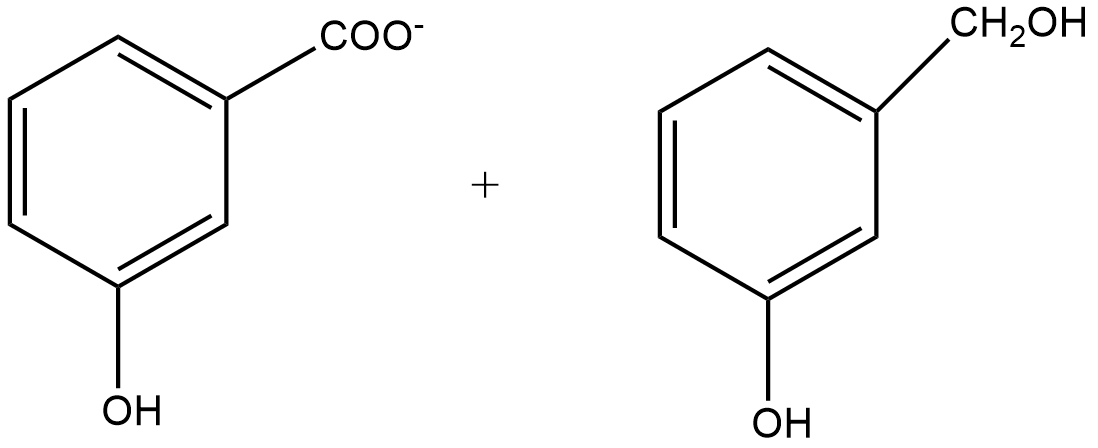
B.
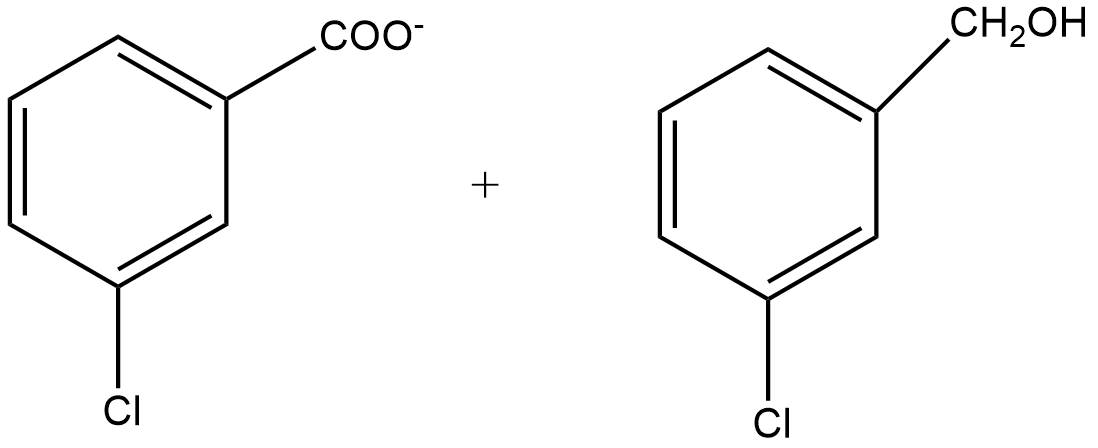
C.
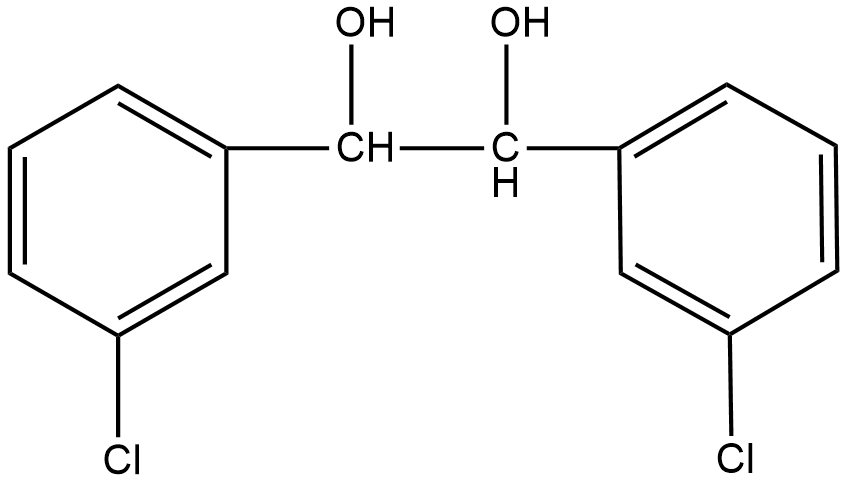
D.
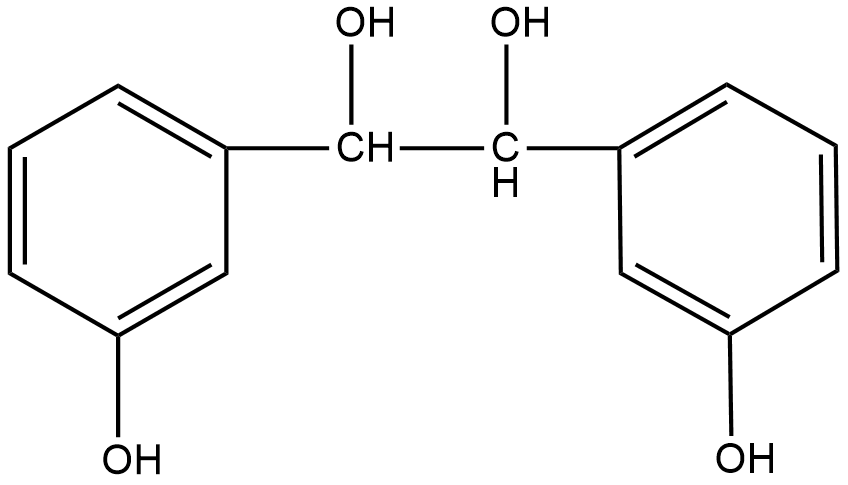




Answer
573.6k+ views
Hint: When aldehyde not containing α-hydrogen reacts with a 50% alkali solution, it undergoes a disproportionation reaction i.e. self oxidation-reduction. As a result, alcohol and acid are formed and the reaction is known as the Cannizzaro reaction.
Complete step by step solution:
Since m-chlorobenzaldehyde does not contain α hydrogen thus with 50% $KOH$ it undergoes the Cannizzaro reaction. The Cannizzaro reaction for the m-chlorobenzaldehyde is given as:
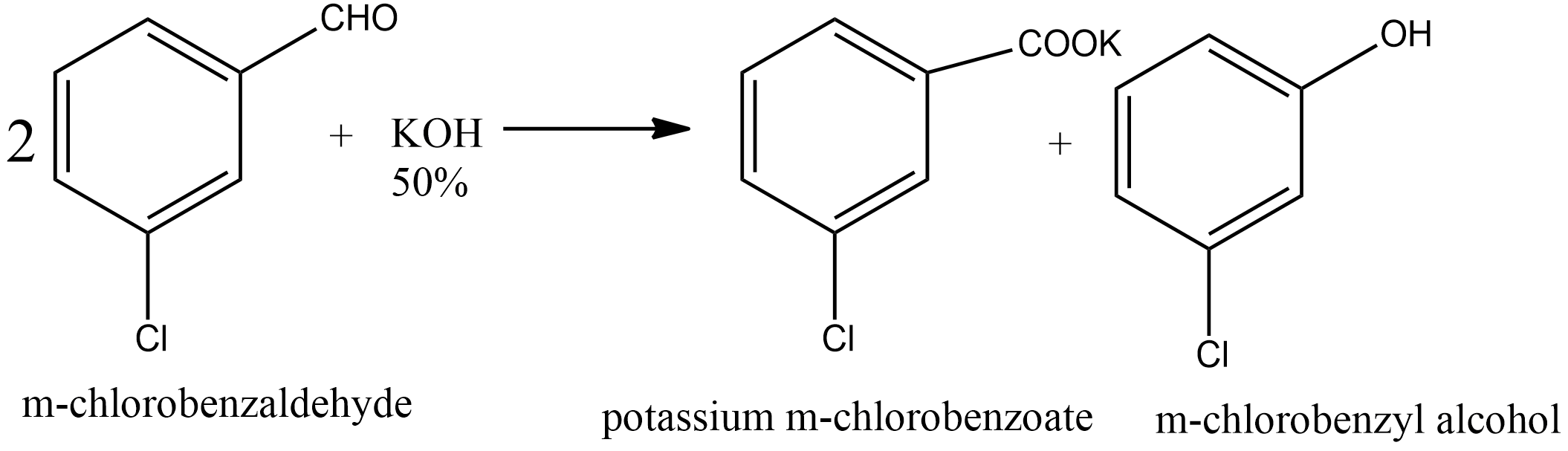
Since it is a disproportionation reaction so one molecule of m-chlorobenzaldehyde is reduced to m-chlorobenzyl alcohol at the cost of others which is oxidized potassium m-chlorobenzoate.
Mechanism of the reaction: Cannizzaro reaction is an example of a hydride transfer reaction which is shown below:
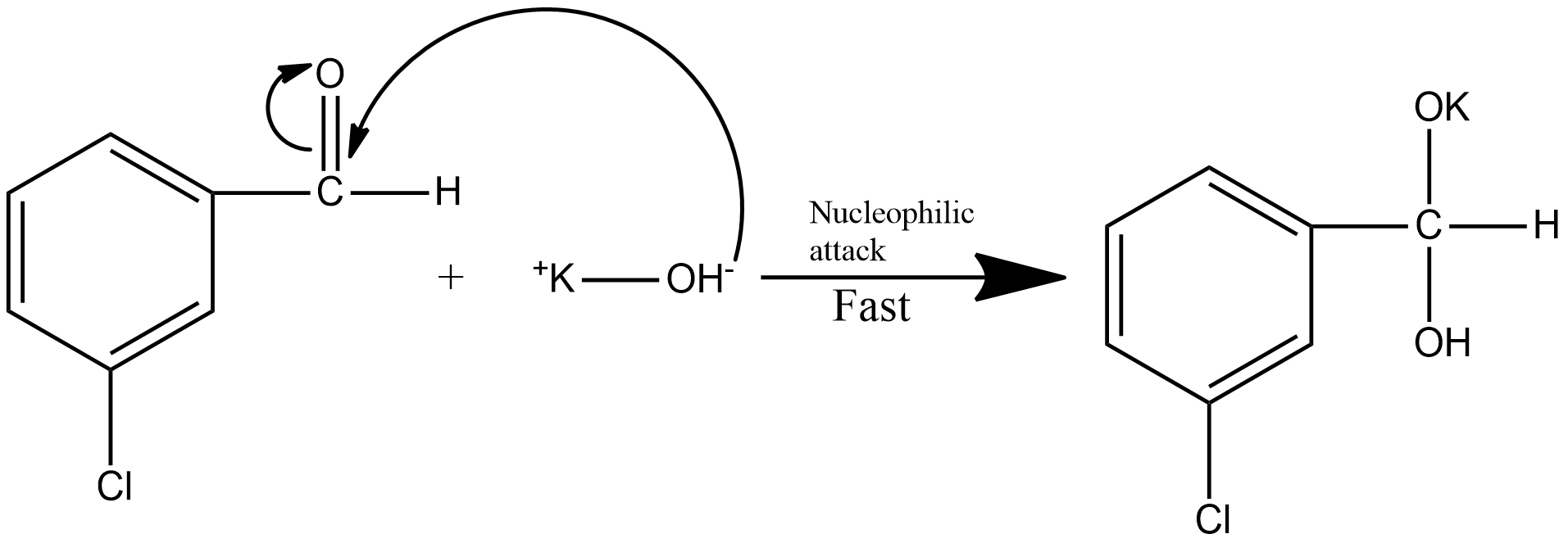
Step1: the first step of the reaction involves the nucleophilic attack. This is the fast step of the mechanism. The nucleophilic attack on the m-chlorobenzaldehyde is shown as:
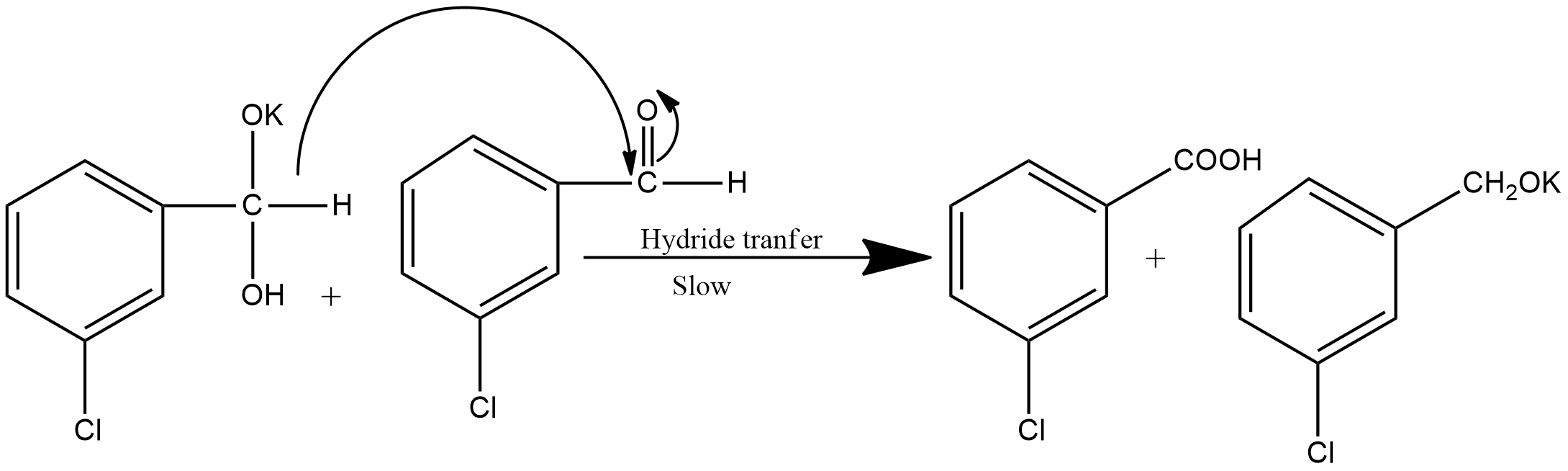
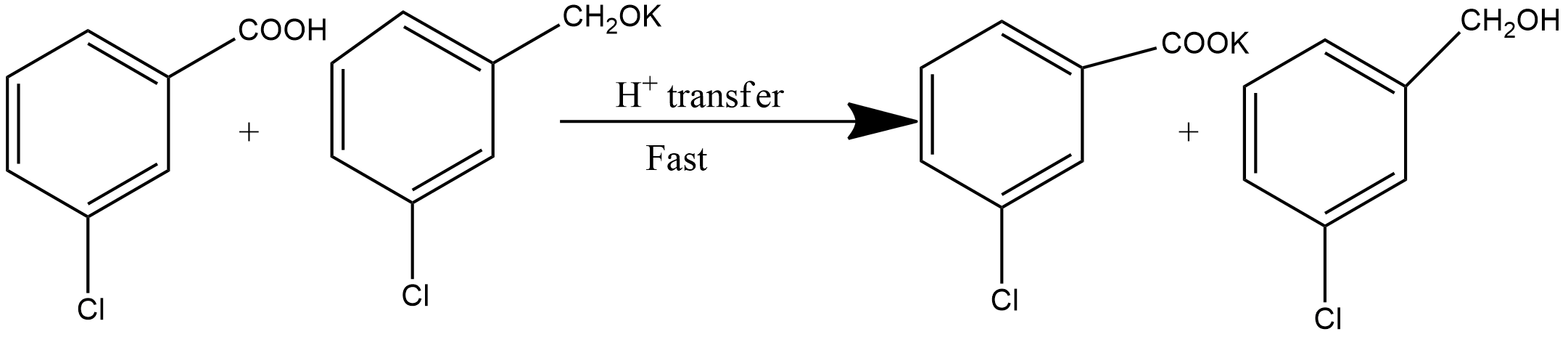
Step2: This step involves the hydride transfer. This is the slowest step hence the rate-determining step of the reaction. The hydride transfer is shown as:
So, the correct answer is Option B.
Note: The products formed in a Cannizzaro reaction include primary alcohols and carboxylic acids and the aldehydes used are used and converted to products. Also, this reaction is known as redox reaction as in the case of the hydride transfer step, one aldehyde oxides to give carboxylic acid whereas another aldehyde reduces to yield alcohol. Also, the hydride transfer is a slow process and hence it is a rate-determining step. At ideal conditions, only 50% of required products are obtained, hence we use crossed Cannizzaro reaction to improve the product yield.
Complete step by step solution:
Since m-chlorobenzaldehyde does not contain α hydrogen thus with 50% $KOH$ it undergoes the Cannizzaro reaction. The Cannizzaro reaction for the m-chlorobenzaldehyde is given as:

Since it is a disproportionation reaction so one molecule of m-chlorobenzaldehyde is reduced to m-chlorobenzyl alcohol at the cost of others which is oxidized potassium m-chlorobenzoate.
Mechanism of the reaction: Cannizzaro reaction is an example of a hydride transfer reaction which is shown below:
Step1: the first step of the reaction involves the nucleophilic attack. This is the fast step of the mechanism. The nucleophilic attack on the m-chlorobenzaldehyde is shown as:

Step2: This step involves the hydride transfer. This is the slowest step hence the rate-determining step of the reaction. The hydride transfer is shown as:


So, the correct answer is Option B.
Note: The products formed in a Cannizzaro reaction include primary alcohols and carboxylic acids and the aldehydes used are used and converted to products. Also, this reaction is known as redox reaction as in the case of the hydride transfer step, one aldehyde oxides to give carboxylic acid whereas another aldehyde reduces to yield alcohol. Also, the hydride transfer is a slow process and hence it is a rate-determining step. At ideal conditions, only 50% of required products are obtained, hence we use crossed Cannizzaro reaction to improve the product yield.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




