
Malonic acid and succinic acid are distinguish by:
A. heating
B. $NaHC{O_3}$
C. both A and B
D. none of these
Answer
538.8k+ views
Hint: Malonic acid has its name originated from a Greek word that means ‘Apple’, it is a dicarboxylic acid found in apples, Succinic acid derived its name from the Latin word succinum which means amber, it exists in living organisms in its dianionic form aptly known as succinate. They both fall under the banner of dicarboxylic acids.
Complete step by step answer:
Malonic acid and succinic acids are distinguished by heating. Malonic acid contains a carbonyl group in beta position. Hence, it undergoes decarboxylation (decarboxylation means removal of $C{O_2}$)
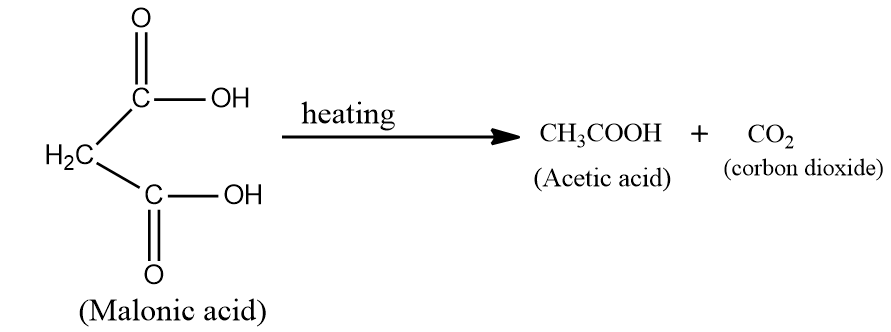
On heating malonic acid, it gives acetic acid $\left( {C{H_3}COOH} \right)$ and carbon dioxide $\left( {C{O_2}} \right)$
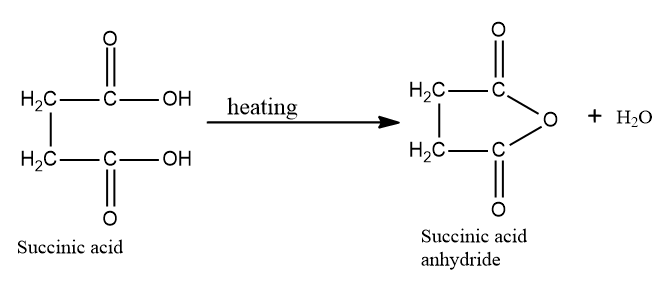
In succinic acid, $\beta - $ carbonyl group is not present and it does not undergo decarboxylation.
So, the correct answer is Option A.
Additional Information:
Malonic acid is used as a building block chemical to produce numerous valuable compounds, the ionised form of malonic acid, as well as its esters and salts, are known as malonates. Succinic acid readily ionizes to form its conjugate base and it is one of the natural acids found in foods such as broccoli, rhubarb, sugar beets, various cheeses, etc.
Note: When facing problems like these a fair knowledge of the molecules structure beforehand is really necessary if we want to solve the given problem accurately and to know or talk about structure a lot of practise is required, so it is always better to do as many problems of this kind as you can so as to inculcate a knowledge vast enough to help you in exams.
Complete step by step answer:
Malonic acid and succinic acids are distinguished by heating. Malonic acid contains a carbonyl group in beta position. Hence, it undergoes decarboxylation (decarboxylation means removal of $C{O_2}$)

On heating malonic acid, it gives acetic acid $\left( {C{H_3}COOH} \right)$ and carbon dioxide $\left( {C{O_2}} \right)$
In succinic acid, $\beta - $ carbonyl group is not present and it does not undergo decarboxylation.

So, the correct answer is Option A.
Additional Information:
Malonic acid is used as a building block chemical to produce numerous valuable compounds, the ionised form of malonic acid, as well as its esters and salts, are known as malonates. Succinic acid readily ionizes to form its conjugate base and it is one of the natural acids found in foods such as broccoli, rhubarb, sugar beets, various cheeses, etc.
Note: When facing problems like these a fair knowledge of the molecules structure beforehand is really necessary if we want to solve the given problem accurately and to know or talk about structure a lot of practise is required, so it is always better to do as many problems of this kind as you can so as to inculcate a knowledge vast enough to help you in exams.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




