
Maximum number of H-atoms that can be exchanged with deuterium in the below compounds?
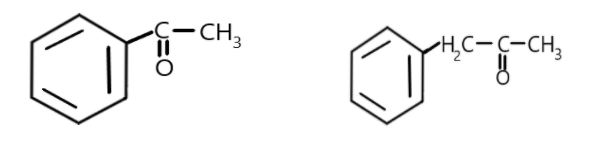

Answer
573k+ views
Hint: As we know that due to the acidic nature of hydrogen they can be replaced by the deuterium by the reaction with heavy water. This reaction requires addition of an acid or base in the presence of excess of heavy water resulting in complete exchange of alpha hydrogen atoms with deuterium.
Complete step by step solution: As we know that due to the acidic nature of hydrogen they can be replaced by the deuterium by the reaction with heavy water. This reaction requires addition of an acid or base in the presence of excess of heavy water resulting in complete exchange of alpha hydrogen atoms with deuterium. First the enolate formation takes place when deuterium will attack the alpha hydrogen which will result in formation of a double bond between carbon-carbon and making the oxygen partially negative.
Then the negatively charged oxygen will try to stabilise by giving back the electrons to bond between oxygen and carbon and the double bond formed will attack the deuterium resulting in attachment of deuterium with carbon.
Similarly in the given option, we can say that oxygen will attack the alpha- hydrogen and result in enolate formation:
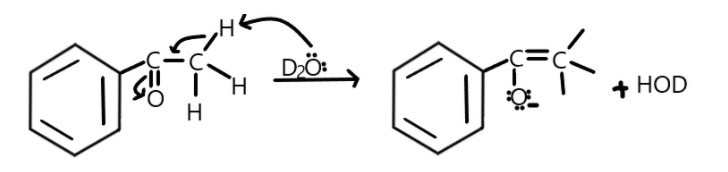
Now the oxygen will pass the electrons to bond between itself and carbon and double bond will attack the deuterium in heavy water as shown:
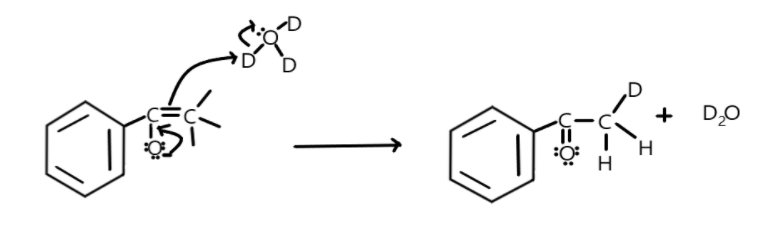
Similarly, all the three alpha-hydrogen can be exchanged by deuterium. So a total of three alpha-hydrogen are exchanged in this reactant.
In the second option the alpha-carbon is the one attached to the first carbon of benzene and it contains two alpha-hydrogens hence, a total of two alpha hydrogens will be exchanged with deuterium as:
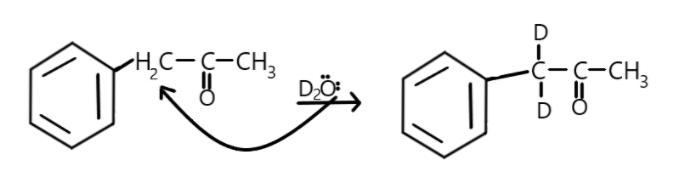
Hence, maximum number of hydrogen atoms exchanged with deuterium is 5.
Note: Remember that the reactivity of benzene decreases in addition to deuterium to the compound in place of hydrogen atoms because the bond strength becomes stronger between deuterium and carbon which is stronger than the bond strength between hydrogen and carbon.
Complete step by step solution: As we know that due to the acidic nature of hydrogen they can be replaced by the deuterium by the reaction with heavy water. This reaction requires addition of an acid or base in the presence of excess of heavy water resulting in complete exchange of alpha hydrogen atoms with deuterium. First the enolate formation takes place when deuterium will attack the alpha hydrogen which will result in formation of a double bond between carbon-carbon and making the oxygen partially negative.
Then the negatively charged oxygen will try to stabilise by giving back the electrons to bond between oxygen and carbon and the double bond formed will attack the deuterium resulting in attachment of deuterium with carbon.
Similarly in the given option, we can say that oxygen will attack the alpha- hydrogen and result in enolate formation:

Now the oxygen will pass the electrons to bond between itself and carbon and double bond will attack the deuterium in heavy water as shown:

Similarly, all the three alpha-hydrogen can be exchanged by deuterium. So a total of three alpha-hydrogen are exchanged in this reactant.
In the second option the alpha-carbon is the one attached to the first carbon of benzene and it contains two alpha-hydrogens hence, a total of two alpha hydrogens will be exchanged with deuterium as:

Hence, maximum number of hydrogen atoms exchanged with deuterium is 5.
Note: Remember that the reactivity of benzene decreases in addition to deuterium to the compound in place of hydrogen atoms because the bond strength becomes stronger between deuterium and carbon which is stronger than the bond strength between hydrogen and carbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




