
What is meant by 'doping' in a semiconductor?
Answer
568.8k+ views
Hint:
There are two types of semiconductors: intrinsic semiconductors which are free of topping and other one is extrinsic semiconductors which are dropped or some impurities are added in them.
Complete step by step answer:
We know that semiconductors are the materials whose properties are Intermediate of insulators and conductors. The same common semiconductors are germanium and silicon.
Doping of semiconductors implies the addition of some impurities in the intrinsic semiconductor to make it extrinsic.
The added impurities are termed as Dopants.
Based on the type of impurities added, the extrinsic semiconductors are divided into two types:
(a)n-type semiconductors
(b)p-type semiconductors
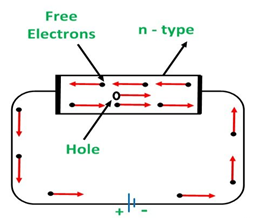
a) n-type semiconductors:
In this semiconductor, doping is done with a pentavalent Dopant such as arsenic. The pentavalent dopant increases the number of free electrons in them.
Due to the increasing number of free electrons in the semiconductors, it will make an effect on the Conduction.
When a voltage is applied on the semiconductors, all the free electrons would move towards the positive terminal as shown in figure.
n-type:
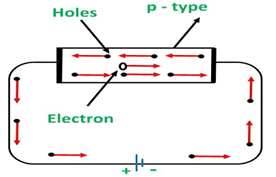
b) p-type semiconductors:
In the semiconductor, doping is done with a trivalent dopant such as gallium and indium.
Due to presence of only three valence electron, the trivalent dopant will form only three covalent bonds with the atoms of semiconductor and hole will be formed as the place of forth covalent bond
When voltage is applied on P type semiconductors, the holes keep shifting from one covalent bond to another covalent bond as shown in figure.
p-type:
In natural or intrinsic state, the semiconductors are poor in conductivity but on doping, due to formation of holes and presence of free electrons, the current flow in them increases and they become better conductors.
Note: Generally, the group \[14\] elements are used for semiconductors formation because they have a valency \[\;4\] electrons due to which they can easily lose or gain the electrons.
The semiconductors are used in the manufacturing of laptops, cell phones, solar panels, diodes, electric circuits etc.
There are two types of semiconductors: intrinsic semiconductors which are free of topping and other one is extrinsic semiconductors which are dropped or some impurities are added in them.
Complete step by step answer:
We know that semiconductors are the materials whose properties are Intermediate of insulators and conductors. The same common semiconductors are germanium and silicon.
Doping of semiconductors implies the addition of some impurities in the intrinsic semiconductor to make it extrinsic.
The added impurities are termed as Dopants.
Based on the type of impurities added, the extrinsic semiconductors are divided into two types:
(a)n-type semiconductors
(b)p-type semiconductors
a) n-type semiconductors:
In this semiconductor, doping is done with a pentavalent Dopant such as arsenic. The pentavalent dopant increases the number of free electrons in them.
Due to the increasing number of free electrons in the semiconductors, it will make an effect on the Conduction.
When a voltage is applied on the semiconductors, all the free electrons would move towards the positive terminal as shown in figure.
n-type:

b) p-type semiconductors:
In the semiconductor, doping is done with a trivalent dopant such as gallium and indium.
Due to presence of only three valence electron, the trivalent dopant will form only three covalent bonds with the atoms of semiconductor and hole will be formed as the place of forth covalent bond
When voltage is applied on P type semiconductors, the holes keep shifting from one covalent bond to another covalent bond as shown in figure.
p-type:

In natural or intrinsic state, the semiconductors are poor in conductivity but on doping, due to formation of holes and presence of free electrons, the current flow in them increases and they become better conductors.
Note: Generally, the group \[14\] elements are used for semiconductors formation because they have a valency \[\;4\] electrons due to which they can easily lose or gain the electrons.
The semiconductors are used in the manufacturing of laptops, cell phones, solar panels, diodes, electric circuits etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




