
What is meant by imine? Give an example of reaction (with mechanism) showing its formation.
Answer
575.7k+ views
Hint: Imine is a product which is obtained by reaction of two types of organic compounds. It is formed as the respective reagent group gets protonated, due to the presence of acid. They are used for alkaloid synthesis.
Complete answer:
In order to answer our question, we need to study about the properties of aldehydes and ketones. Due to the polar nature of the carbonyl group, nucleophilic addition reactions are quite common to these compounds in which nucleophile attacks first followed by attack of electrophile. If attacking nucleophiles are weak, reaction is usually carried out in a weakly acidic medium. These are called acid catalysed nucleophilic addition reactions. The factors which help us determine reactivity are:
Steric factor: Attack of nucleophile changes the trigonal planar carbonyl group to tetrahedral state. Increase in size of alkyl or aryl groups around carbon atoms produce steric hindrance to the attacking nucleophile. Greater the number of groups, more the hindrance and lesser will be the tendency of nucleophiles to attack the carbon atom.
Electronic factor: Alkyl groups present on the carbonyl group show +I effect and tend to increase electron density on it, due to which the attacking tendency of nucleophile decreases. It means, more the +I effect, lesser will be the reactivity of carbonyl compounds.
Now, let us come to our question. Ammonia and its derivatives add to the carbonyl group of aldehydes and ketones. It is an acid catalysed reversible reaction. The product formed in this reaction is called an imine. The mechanism is:
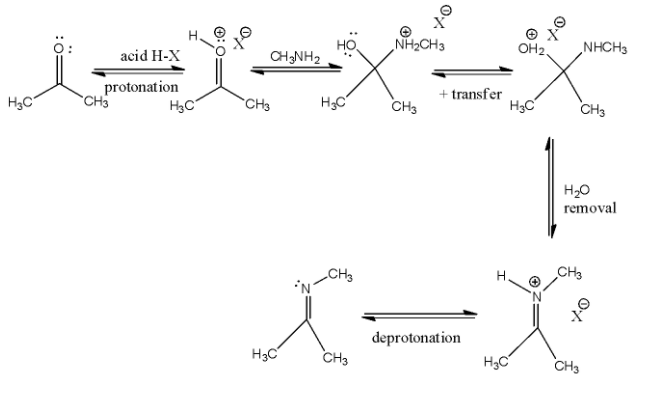
Firstly, protonation takes place via the acid and then the ammoniacal derivative is reacted. One more proton transfer takes place after which water gets removed and deprotonation takes place, in order to get our final product.
Note:
During the formation of imine, the medium used is acid medium so that protonation takes place. The equilibrium favours the product formation due to rapid dehydration of intermediate to form $(-C=N-Z)$. Here Z can be alkyl as well as aryl group.
Complete answer:
In order to answer our question, we need to study about the properties of aldehydes and ketones. Due to the polar nature of the carbonyl group, nucleophilic addition reactions are quite common to these compounds in which nucleophile attacks first followed by attack of electrophile. If attacking nucleophiles are weak, reaction is usually carried out in a weakly acidic medium. These are called acid catalysed nucleophilic addition reactions. The factors which help us determine reactivity are:
Steric factor: Attack of nucleophile changes the trigonal planar carbonyl group to tetrahedral state. Increase in size of alkyl or aryl groups around carbon atoms produce steric hindrance to the attacking nucleophile. Greater the number of groups, more the hindrance and lesser will be the tendency of nucleophiles to attack the carbon atom.
Electronic factor: Alkyl groups present on the carbonyl group show +I effect and tend to increase electron density on it, due to which the attacking tendency of nucleophile decreases. It means, more the +I effect, lesser will be the reactivity of carbonyl compounds.
Now, let us come to our question. Ammonia and its derivatives add to the carbonyl group of aldehydes and ketones. It is an acid catalysed reversible reaction. The product formed in this reaction is called an imine. The mechanism is:

Firstly, protonation takes place via the acid and then the ammoniacal derivative is reacted. One more proton transfer takes place after which water gets removed and deprotonation takes place, in order to get our final product.
Note:
During the formation of imine, the medium used is acid medium so that protonation takes place. The equilibrium favours the product formation due to rapid dehydration of intermediate to form $(-C=N-Z)$. Here Z can be alkyl as well as aryl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




