
What is meant by Oxime? Give an example of reaction (with mechanism) showing its formation.
Answer
582.3k+ views
Hint: We know that, a functional group that possesses a double bond of C and N is termed as imine. The nitrogen atom further can be bonded to an organic group (R) or to a hydrogen(H). It is considered a Schiff base if the group to which the N atom is bonded is not a hydrogen atom. Oxime is a compound that belongs to this class.
Complete step by step answer: Let’s understand oxime and its structure.
The chemical compounds that belong to the imine class are known as oximes. The general formula of oxime is ${{\rm{R}}_{\rm{1}}}{{\rm{R}}_{\rm{2}}}{\rm{C}} = {\rm{NOH}}$. Where, ${{\rm{R}}_{\rm{1}}}$ is an alkyl group and ${{\rm{R}}_2}$is hydrogen. Oxime can be prepared by the reaction of hydroxylamine, an aldehyde and a ketone. The structure of oxime is,
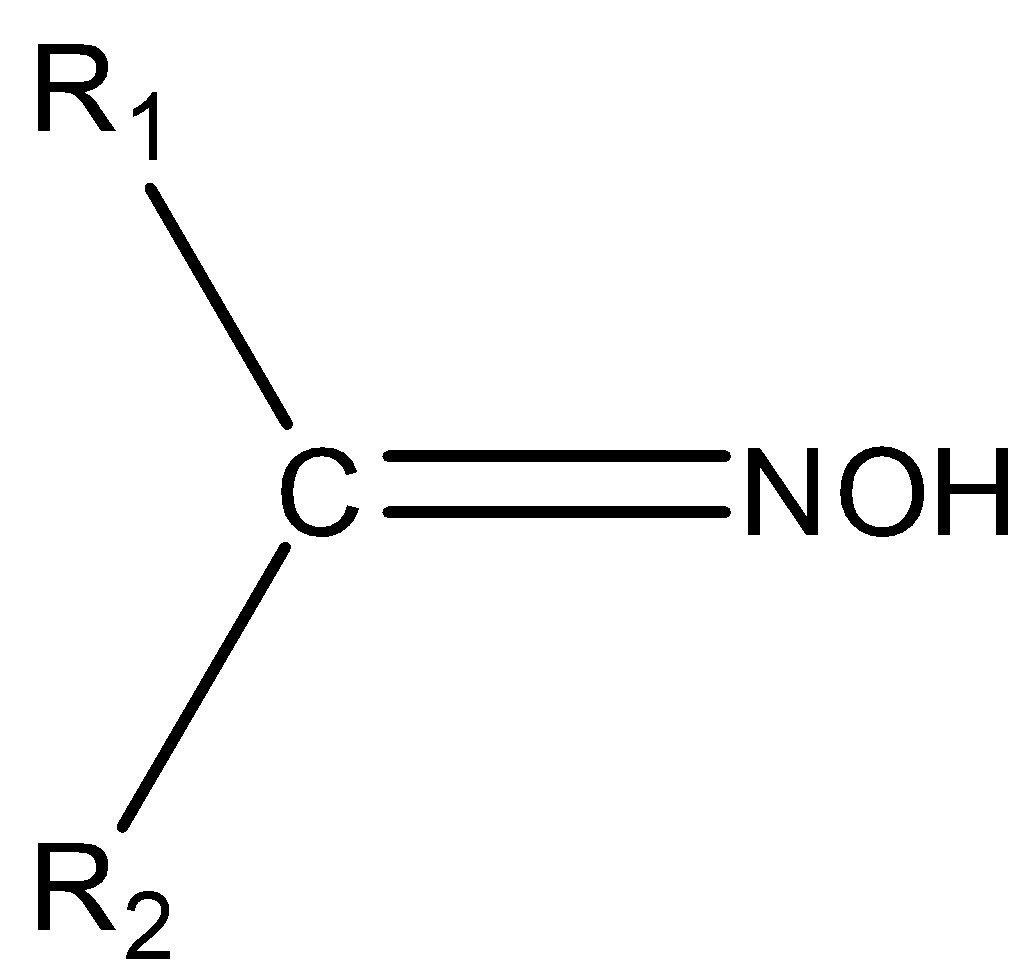
So, we can say that its structure consists of two side chains consisting of carbon as the central atom. Some examples of oxime are aldoxime, aldicarb oxime, ketoxime etc.
Now, we will understand the formation of oxime with the help of mechanisms. We know that, reaction of ketone and aldehyde with hydroxylamine $\left( { - {\rm{N}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}} \right)$ results oxime.
Step 1: The nucleophilic attack of nitrogen of hydroxylamine to the carbonyl carbon of the aldehyde.
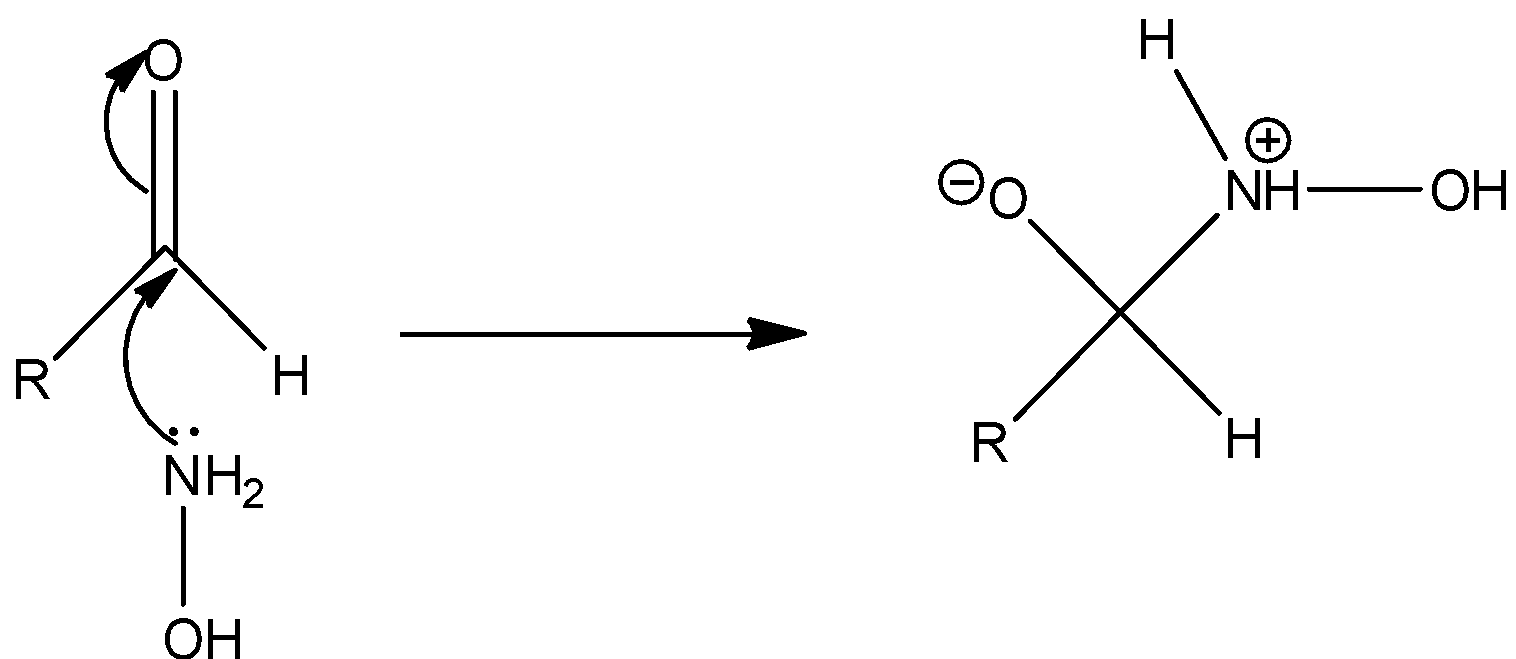
Step 2: The negatively charged oxygen takes up a proton and thus proton transfer takes place.
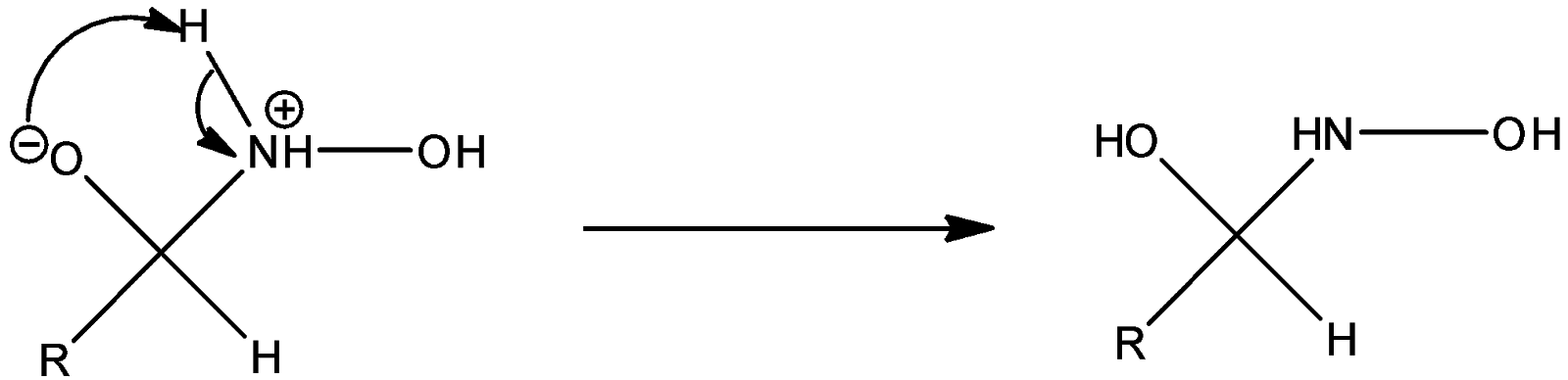
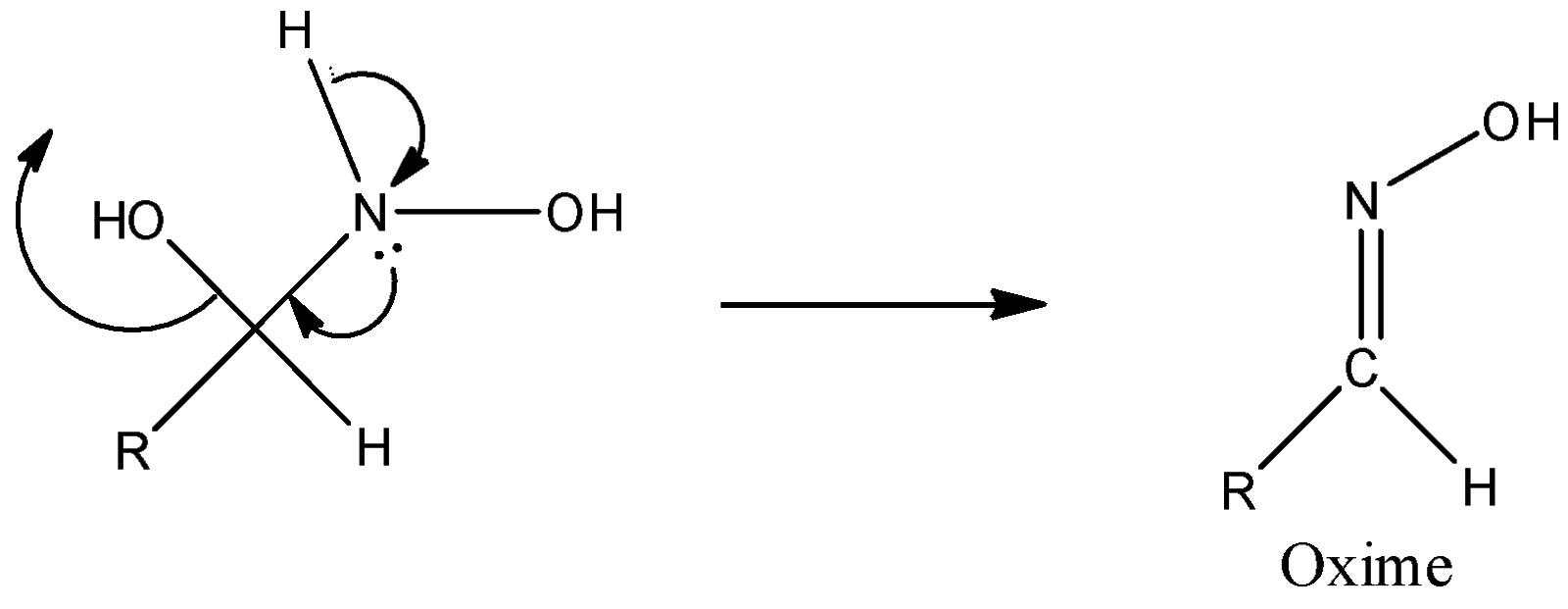
Step 3: Successive proton transfers result in formation of oxime.
Note: There are many industrial uses of oxime, such as, production of caprolactam (monomer of nylon-6), catalytic uses in industry etc. Use of compounds of oxime as antidotes is also one of its uses. Acetone oxime is also used as a corrosion inhibitor.
Complete step by step answer: Let’s understand oxime and its structure.
The chemical compounds that belong to the imine class are known as oximes. The general formula of oxime is ${{\rm{R}}_{\rm{1}}}{{\rm{R}}_{\rm{2}}}{\rm{C}} = {\rm{NOH}}$. Where, ${{\rm{R}}_{\rm{1}}}$ is an alkyl group and ${{\rm{R}}_2}$is hydrogen. Oxime can be prepared by the reaction of hydroxylamine, an aldehyde and a ketone. The structure of oxime is,

So, we can say that its structure consists of two side chains consisting of carbon as the central atom. Some examples of oxime are aldoxime, aldicarb oxime, ketoxime etc.
Now, we will understand the formation of oxime with the help of mechanisms. We know that, reaction of ketone and aldehyde with hydroxylamine $\left( { - {\rm{N}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}} \right)$ results oxime.
Step 1: The nucleophilic attack of nitrogen of hydroxylamine to the carbonyl carbon of the aldehyde.

Step 2: The negatively charged oxygen takes up a proton and thus proton transfer takes place.

Step 3: Successive proton transfers result in formation of oxime.

Note: There are many industrial uses of oxime, such as, production of caprolactam (monomer of nylon-6), catalytic uses in industry etc. Use of compounds of oxime as antidotes is also one of its uses. Acetone oxime is also used as a corrosion inhibitor.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




