
What is meant by the term coordination number in solids? What is the coordination number in a face centered cubic close packing?
Answer
596.7k+ views
Hint:We know that this question is taken from the chapter of solid state. And coordination numbers we can find by counting the number of atoms attached with a single atom.
Complete step by step solution:
> The number of atoms nearest to an atom in the crystalline structure is called the coordination number. Coordination number, also called Ligancy, the number of atoms, ions, or molecules that a central atom or ion holds as its nearest neighbours in a complex or coordination compound or in a crystal.
> The term "closest packed structures" refers to the most tightly packed or space-efficient composition of crystal structures (lattices).
- A unit cell is the smallest representation of an entire crystal. All crystal lattices are built of repeating unit cells.
- The face-centered cubic (fcc) has a coordination number of 12 and contains 4 atoms per unit cell. Let’s take an atom at the corner, this corner atom is attached with 6 other atoms of the corner and 6 atoms of face.
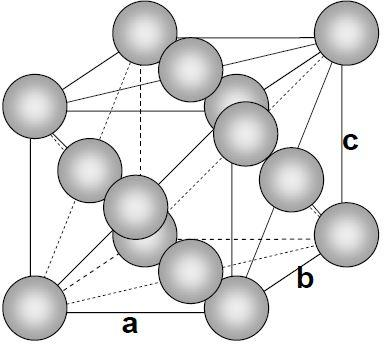
Note: The packing of spheres can describe the solid structures of crystals. In a crystal structure, the centers of atoms, ions, or molecules lie on the lattice points.
Complete step by step solution:
> The number of atoms nearest to an atom in the crystalline structure is called the coordination number. Coordination number, also called Ligancy, the number of atoms, ions, or molecules that a central atom or ion holds as its nearest neighbours in a complex or coordination compound or in a crystal.
> The term "closest packed structures" refers to the most tightly packed or space-efficient composition of crystal structures (lattices).
- A unit cell is the smallest representation of an entire crystal. All crystal lattices are built of repeating unit cells.
- The face-centered cubic (fcc) has a coordination number of 12 and contains 4 atoms per unit cell. Let’s take an atom at the corner, this corner atom is attached with 6 other atoms of the corner and 6 atoms of face.

Note: The packing of spheres can describe the solid structures of crystals. In a crystal structure, the centers of atoms, ions, or molecules lie on the lattice points.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




