
How is the mechanism of reduction of benzophenone to diphenylmethanol?
Answer
552.6k+ views
Hint: Reduction is defined as the loss of electrons. The reduction reaction is the major reaction used to reduce the double bond into single. The benzophenone is reduced to diphenylmethanol using a reducing agent like sodium borohydride.
Complete step by step answer:
The reduction reaction is one of the major classes of reaction used in organic chemistry. Reduction reaction is the opposite of oxidation reaction which is determined by the loss of oxygen from a bond, addition of hydrogen to a bond or replacement of more electronegative atoms by the less electronegative atom. The best method of reduction is hydrogenation reaction which reduces the double bonds into single.
The benzophenone is reduced to diphenylmethanol by using sodium borohydride. The sodium borohydride is used as the reducing agent.
The reaction is shown below.
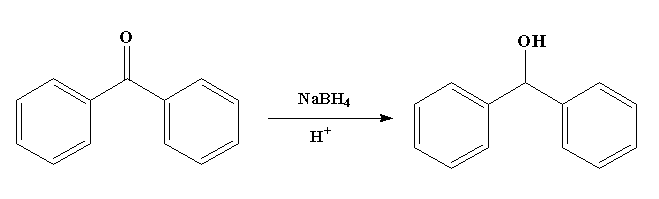
In this reaction, benzophenone is converted to diphenylmethanol with the help of sodium borohydride
The sodium borohydride contains a sodium ion $N{a^ + }$ and borohydride ion $BH_4^ - $. The negative hydride acts as a nucleophile and attacks the carbonyl carbon of the benzophenone and due to this the shifting of bond takes place and the negative charge is generated on the oxygen atom. Then the negative charge of the oxygen attacks and abstract the hydrogen ion to generate hydroxide to form diphenylmethanol.
Note: There is a difference between the hydrogen ion and hydride ion. Hydrogen ion is the cation and hydride ion is the anion. The other reducing agents apart from sodium borohydride is zinc amalgam, Lithium aluminum hydride, diborane, sodium amalgam.
Complete step by step answer:
The reduction reaction is one of the major classes of reaction used in organic chemistry. Reduction reaction is the opposite of oxidation reaction which is determined by the loss of oxygen from a bond, addition of hydrogen to a bond or replacement of more electronegative atoms by the less electronegative atom. The best method of reduction is hydrogenation reaction which reduces the double bonds into single.
The benzophenone is reduced to diphenylmethanol by using sodium borohydride. The sodium borohydride is used as the reducing agent.
The reaction is shown below.

In this reaction, benzophenone is converted to diphenylmethanol with the help of sodium borohydride
The sodium borohydride contains a sodium ion $N{a^ + }$ and borohydride ion $BH_4^ - $. The negative hydride acts as a nucleophile and attacks the carbonyl carbon of the benzophenone and due to this the shifting of bond takes place and the negative charge is generated on the oxygen atom. Then the negative charge of the oxygen attacks and abstract the hydrogen ion to generate hydroxide to form diphenylmethanol.
Note: There is a difference between the hydrogen ion and hydride ion. Hydrogen ion is the cation and hydride ion is the anion. The other reducing agents apart from sodium borohydride is zinc amalgam, Lithium aluminum hydride, diborane, sodium amalgam.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




