
Mesomeric effect involves the delocalization of:
A) \[\pi \] electrons
B) \[\sigma \] electrons
C) Protons
D) None of these
Answer
558.9k+ views
Hint: Mesomeric effect is also called resonance effect and its types depend on the nature of the substituent present in the compound. There are two types of mesomeric effect which are negative mesomeric effect and positive mesomeric effect.The mesomeric effect is the permanent effect.
Complete step-by-step answer:
The effect in which the attached substituent generates the polarity between two pi bonds or pi bond and lone pair electrons is known as the mesomeric effect.
There are two types of mesomeric effect 1) positive mesomeric effect and 2) negative mesomeric effect.
Groups that can donate the electron show a positive mesomeric effect.\[{\text{ - N}}{{\text{H}}_{\text{2}}}{\text{, - NHR, - OR, - }}\,{\text{OCOR, - Cl, - Br, - I, - F}}\,{\text{etc}}\], here, we can see that all electron releasing groups show a positive mesomeric effect.
Here. The example of the positive mesomeric effect is as follows:
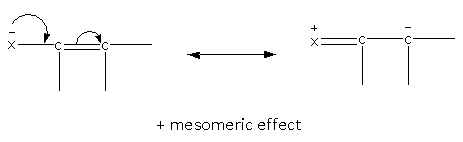
Here, X is the electron-donating group.
Groups that can withdraw the electron shows a negative mesomeric effect.\[{\text{ - N}}{{\text{O}}_{\text{2}}}{\text{, - CN, - COH, - }}\,{\text{CO, - COOH, - COOR}}\,\,{\text{etc}}\], all these are electron-withdrawing groups.
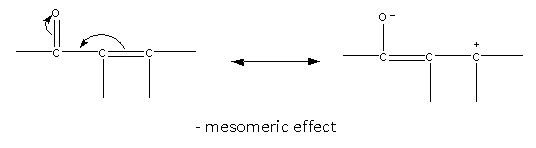
Here, we can see that there is delocalization of pi electrons.
Here, option (A) \[\pi \]electrons which is the correct answer for the given question.
Now, option (B) \[\sigma \]electrons which is an incorrect answer for the given question because in mesomeric effect there is delocalization of the pi electrons are observed.
Option (C) protons is an incorrect answer for the given question.
Option(D) None of these is also an incorrect answer for the given question.
Hence the correct answer is option ‘A’.
Note: Groups that can donate the electron shows a positive mesomeric effect while Groups that can withdraw the electron shows a negative mesomeric effect.
The positive mesomeric effect is represented as +M and the negative mesomeric effect is represented as -M.
Complete step-by-step answer:
The effect in which the attached substituent generates the polarity between two pi bonds or pi bond and lone pair electrons is known as the mesomeric effect.
There are two types of mesomeric effect 1) positive mesomeric effect and 2) negative mesomeric effect.
Groups that can donate the electron show a positive mesomeric effect.\[{\text{ - N}}{{\text{H}}_{\text{2}}}{\text{, - NHR, - OR, - }}\,{\text{OCOR, - Cl, - Br, - I, - F}}\,{\text{etc}}\], here, we can see that all electron releasing groups show a positive mesomeric effect.
Here. The example of the positive mesomeric effect is as follows:

Here, X is the electron-donating group.
Groups that can withdraw the electron shows a negative mesomeric effect.\[{\text{ - N}}{{\text{O}}_{\text{2}}}{\text{, - CN, - COH, - }}\,{\text{CO, - COOH, - COOR}}\,\,{\text{etc}}\], all these are electron-withdrawing groups.

Here, we can see that there is delocalization of pi electrons.
Here, option (A) \[\pi \]electrons which is the correct answer for the given question.
Now, option (B) \[\sigma \]electrons which is an incorrect answer for the given question because in mesomeric effect there is delocalization of the pi electrons are observed.
Option (C) protons is an incorrect answer for the given question.
Option(D) None of these is also an incorrect answer for the given question.
Hence the correct answer is option ‘A’.
Note: Groups that can donate the electron shows a positive mesomeric effect while Groups that can withdraw the electron shows a negative mesomeric effect.
The positive mesomeric effect is represented as +M and the negative mesomeric effect is represented as -M.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




