
Methyl acetylene on ozonolysis gives
(a)Acetaldehyde
(b)Acetone
(c)Gloxal
(d)Methyl glyoxal
Answer
517.5k+ views
Hint: Ozonolysis is an organic reaction where the unsaturated bonds of alkenes, alkynes, or azo compounds are cleaved with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl group while azo compounds form nitrosamines.
Complete answer:
Methyl acetylene on ozonolysis gives acetaldehyde. Therefore, option A is the correct option. The reaction is written below.
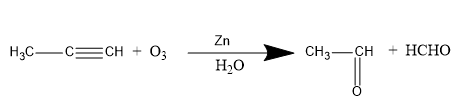
In ozonolysis reaction the formation of intermediate also takes place which is ozonoid. The short method or short trick to learn this reaction is that you have to break the double and triple bond and place the hydrogen with each molecule after breaking the bonds. When the ozonoid is formed this reaction proceeds further in the presence of $Zn$ and ${H_2}O$ to form the desired product. In the above reaction, the products formed are acetaldehyde $\left( {{C_2}{H_4}O} \right)$ and formaldehyde $\left( {HCHO} \right)$. When only ozonolysis of acetylene takes place, the product formed is glyoxal.
Hence the correct answer is option C.
Note:
Ozonolysis is an organic reaction in which the unsaturated bonds of alkenes, alkynes, and azo compounds get broken or cleaved by ozone. In the ozonolysis reaction of alkenes and alkynes formation of organic compounds takes place. In this reaction carbon-carbon multiple bonds have been replaced by the carbonyl group whereas in the ozonolysis of azo compounds, formation of nitrosamines takes place. The outcome just depends on the bonds being oxidized.
Complete answer:
Methyl acetylene on ozonolysis gives acetaldehyde. Therefore, option A is the correct option. The reaction is written below.

In ozonolysis reaction the formation of intermediate also takes place which is ozonoid. The short method or short trick to learn this reaction is that you have to break the double and triple bond and place the hydrogen with each molecule after breaking the bonds. When the ozonoid is formed this reaction proceeds further in the presence of $Zn$ and ${H_2}O$ to form the desired product. In the above reaction, the products formed are acetaldehyde $\left( {{C_2}{H_4}O} \right)$ and formaldehyde $\left( {HCHO} \right)$. When only ozonolysis of acetylene takes place, the product formed is glyoxal.
Hence the correct answer is option C.
Note:
Ozonolysis is an organic reaction in which the unsaturated bonds of alkenes, alkynes, and azo compounds get broken or cleaved by ozone. In the ozonolysis reaction of alkenes and alkynes formation of organic compounds takes place. In this reaction carbon-carbon multiple bonds have been replaced by the carbonyl group whereas in the ozonolysis of azo compounds, formation of nitrosamines takes place. The outcome just depends on the bonds being oxidized.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




