
When methyl group is in axial position in methyl cyclohexane, the molecule has
A. One Gauche interaction
B. Two Gauche interaction
C. No Gauche interaction
D. Three Gauche interaction
Answer
585.3k+ views
Hint: Atoms like space and the closer they come, the more unstable the conformation becomes due to presence of steric hindrance between the atoms. Try to draw the conformational diagram of methyl cyclohexane and find out with how much hydrogen atoms, the methyl group is interacting in the axial position.
Complete step by step answer:
The cyclohexane ring can have many different shapes. A single cyclohexane molecule is in a continuous state of flexing or flipping into different shapes or conformations like chair form (which is more stable), half chair form, twist boat and boat form (least stable). Chair form has no angle strain and the $C-C$ bonds are staggered here.
So, examinations of the chair form of cyclohexane proves that the hydrogen atoms in the structure are divided into two categories. Twelve hydrogens are not structurally equivalent. Six of them are located about a periphery of the carbon ring, and are termed as equatorial. The other six are oriented above and below the approximate plane of the ring, and are termed as axial because they are aligned parallel to the symmetry axis of the ring.
So, because of the axial bonds that parallel to each other, subsequent larger than hydrogen atoms generally suffer greater steric crowding when they are oriented axial rather than equatorial.
Therefore, here as per the given question, when the methyl group (which is bulkier than the hydrogen atom) occupies an axial position, it will suffer steric hindrance by the two axial hydrogens located on the same side of the ring. A careful examination shows that this hindrance is due to gauche-like orientation of the methyl group with the $C-3$ and $C-5$ in the ring. This is shown in the below figure:
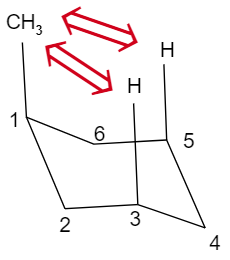
Therefore, we see that it has repulsions from two hydrogen atoms. Thus, it will have two gauche interactions.
Hence, the correct option is B.
Note: A methyl group is larger than a hydrogen atom. Remember when a bulkier group in cyclohexane is present in the axial position, the larger group and the hydrogen atom present in the axial position of the ring will repel each other and these interactions are called as axial-axial interaction or gauche interaction. Whereas when the larger group is in the equatorial position, the repulsions are minimum.
Complete step by step answer:
The cyclohexane ring can have many different shapes. A single cyclohexane molecule is in a continuous state of flexing or flipping into different shapes or conformations like chair form (which is more stable), half chair form, twist boat and boat form (least stable). Chair form has no angle strain and the $C-C$ bonds are staggered here.
So, examinations of the chair form of cyclohexane proves that the hydrogen atoms in the structure are divided into two categories. Twelve hydrogens are not structurally equivalent. Six of them are located about a periphery of the carbon ring, and are termed as equatorial. The other six are oriented above and below the approximate plane of the ring, and are termed as axial because they are aligned parallel to the symmetry axis of the ring.
So, because of the axial bonds that parallel to each other, subsequent larger than hydrogen atoms generally suffer greater steric crowding when they are oriented axial rather than equatorial.
Therefore, here as per the given question, when the methyl group (which is bulkier than the hydrogen atom) occupies an axial position, it will suffer steric hindrance by the two axial hydrogens located on the same side of the ring. A careful examination shows that this hindrance is due to gauche-like orientation of the methyl group with the $C-3$ and $C-5$ in the ring. This is shown in the below figure:

Therefore, we see that it has repulsions from two hydrogen atoms. Thus, it will have two gauche interactions.
Hence, the correct option is B.
Note: A methyl group is larger than a hydrogen atom. Remember when a bulkier group in cyclohexane is present in the axial position, the larger group and the hydrogen atom present in the axial position of the ring will repel each other and these interactions are called as axial-axial interaction or gauche interaction. Whereas when the larger group is in the equatorial position, the repulsions are minimum.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




