
Methyl orange (an acid-base indicator) can be prepared by following a sequence of reactions.
What would be the structure of methyl orange?

Answer
590.4k+ views
Hint: Methyl-orange is an indicator used in acid-base titration. The pH of the methyl orange indicator is 3−5. Methyl orange forms a red colouration in acidic medium but it forms a yellow colouration in basic medium. Means in acid-base titration reaction the color of the solution changed from red to yellow color.
Complete answer:
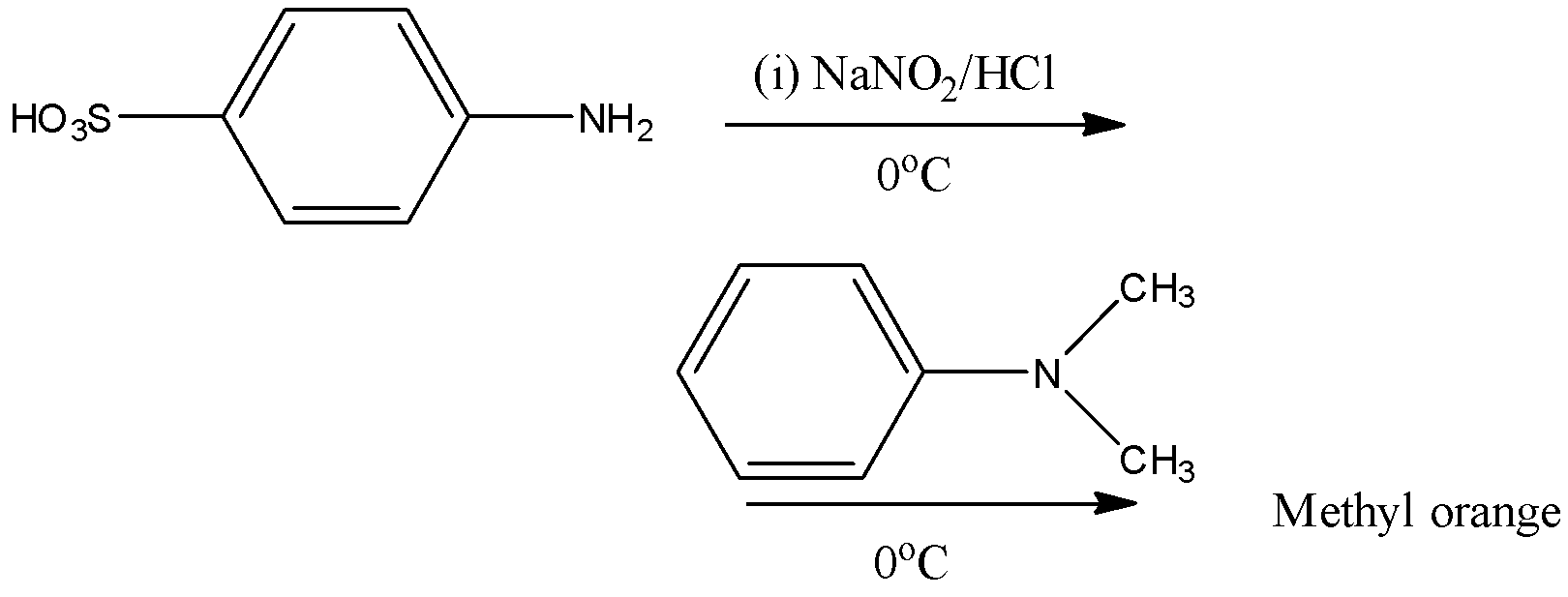
Methyl orange is going to be prepared in two steps.
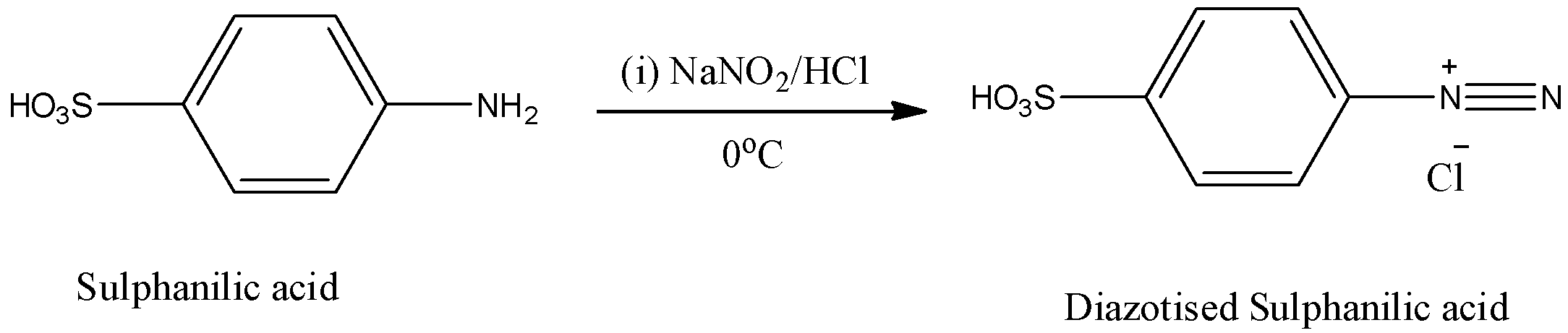
Step-1: Sulphanilic acid reacts with sodium nitrate in presence of hydrochloric acid and forms diazotized sulfanilic acid at \[{{0}^{o}}C\].
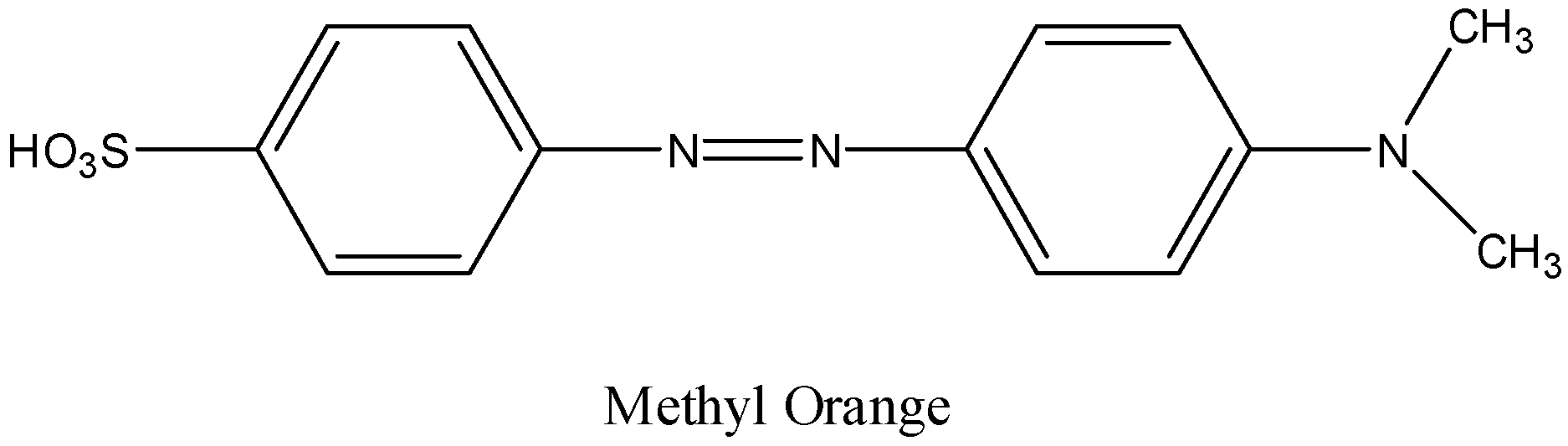
Step-2: The formed diazotized sulfanilic acid reacts with N,N-dimethyl aniline and forms methyl orange as the product.

Therefore the structure of methyl orange is as follows.
Additional information:
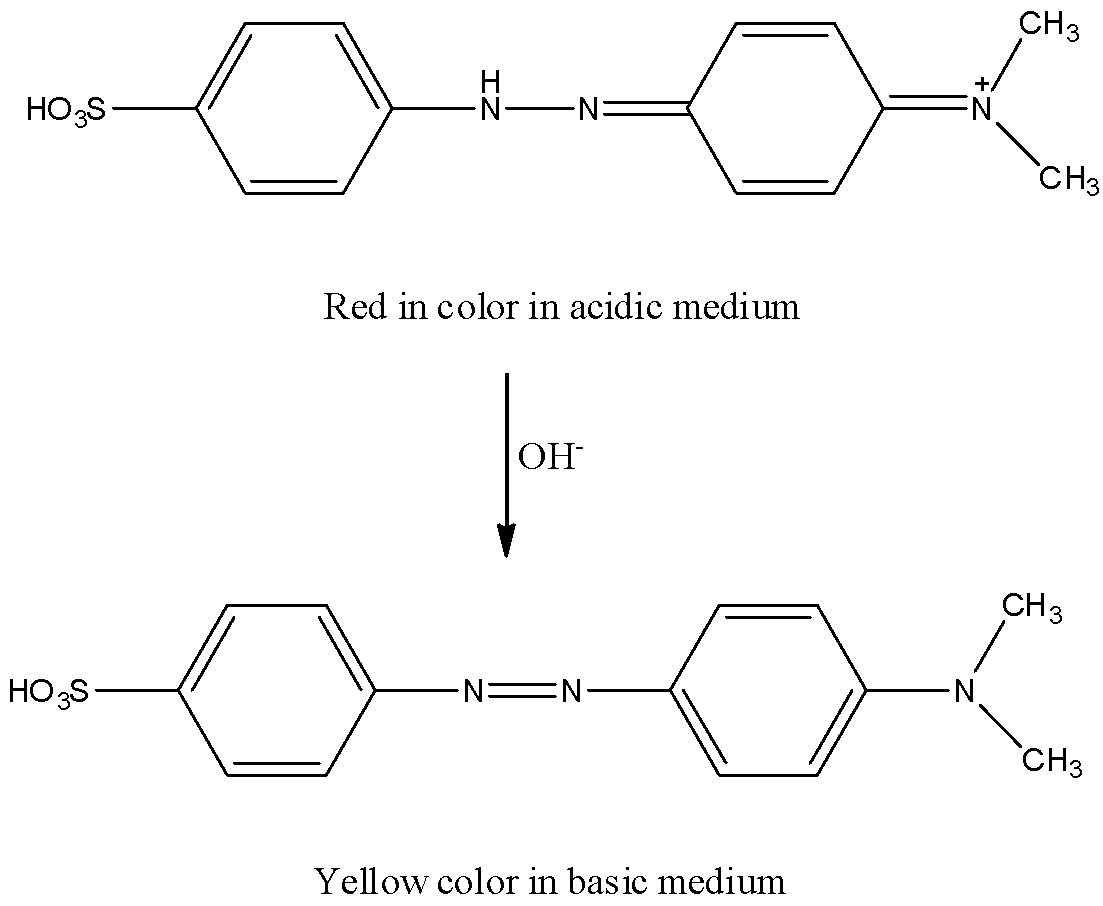
When methyl orange reacts with acid, one of the central nitrogen atoms gets reduced and turns red in color.
The reaction of methyl orange with acid is as follows.
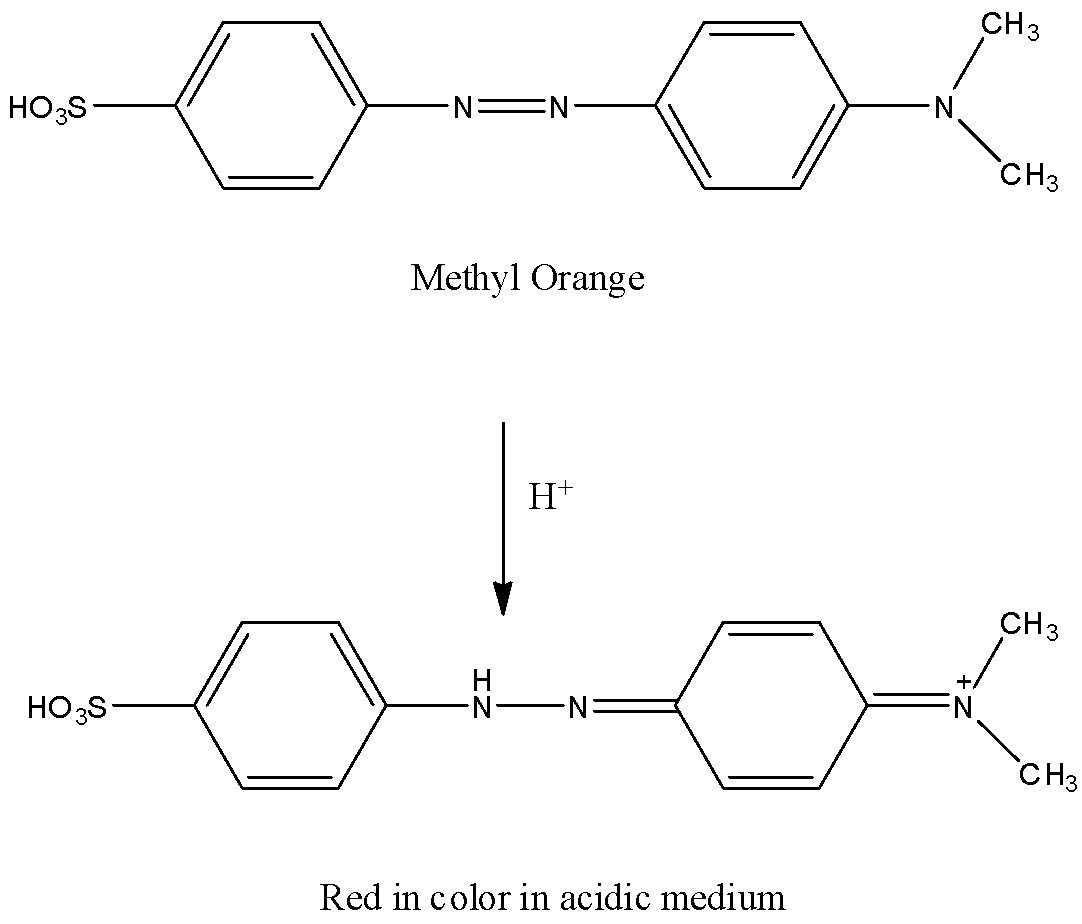
By adding a base the red color changes to yellow.
The reaction is as follows.
Methylorange is very rarely used in textile applications because it is sensitive to acids.
Methyl orange is a strongly colored compound and used in dyeing and in printing textiles.
Note:
Methyl orange absorbs light in the visible range of the electromagnetic spectrum and shows the color change. Methyl orange contains an extended conjugation system of delocalized electrons so it is called chromophore. Chromophore gives color to the compounds.
Complete answer:
Methyl orange is going to be prepared in two steps.
Step-1: Sulphanilic acid reacts with sodium nitrate in presence of hydrochloric acid and forms diazotized sulfanilic acid at \[{{0}^{o}}C\].

Step-2: The formed diazotized sulfanilic acid reacts with N,N-dimethyl aniline and forms methyl orange as the product.

Therefore the structure of methyl orange is as follows.

Additional information:
When methyl orange reacts with acid, one of the central nitrogen atoms gets reduced and turns red in color.
The reaction of methyl orange with acid is as follows.

By adding a base the red color changes to yellow.
The reaction is as follows.

Methylorange is very rarely used in textile applications because it is sensitive to acids.
Methyl orange is a strongly colored compound and used in dyeing and in printing textiles.
Note:
Methyl orange absorbs light in the visible range of the electromagnetic spectrum and shows the color change. Methyl orange contains an extended conjugation system of delocalized electrons so it is called chromophore. Chromophore gives color to the compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




