
Molar mass of glucose is:
(A) 180g
(B) 129g
(C) 190g
(D) 200g
Answer
570.3k+ views
Hint: To answer this question firstly we should know the chemical formula of glucose and secondly, the definition as well as the formula to calculate molar mass. This will help us in solving the question within no time.
Complete answer:
The glucose is a monosaccharide, that is found in free State in fruits and in some parts of the plants. It contains an aldehyde group and a six carbon atom.
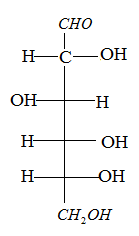
The chemical formula is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$.
Molar mass is the arithmetic sum of atomic mass of elements in the molecule.
Molar mass = $\dfrac{Molecular\text{ }weight\text{ }in\text{ }gram}{1\text{ }mole}$
The mass of a particular atom of an element which is expressed in atomic mass units.
The atomic mass of carbon = 12
The atomic mass of hydrogen =1.008
The atomic weight of oxygen = 16
The molar mass of glucose = atomic mass of carbon$\times $6 + atomic mass of hydrogen$\times $12 + atomic mass of oxygen $\times $ 6
Any number divided by 1 gives the same number.
= 12 $\times $ 6 + 1.008 $\times $12 + 16 $\times $ 6
= 180.096g
So, from the above discussion we can conclude that option A is the correct answer.
Note:
It is very important to be careful while calculating. It is better to remember the atomic mass of elements in periodic tables. If we know the atomic mass of the elements and formula to calculate in hand, solving this question takes less time.180g means there is 180g per mole as the definition molar mass is measured in grams per mole. As we know that any number divided by 1 gives the same number.
Complete answer:
The glucose is a monosaccharide, that is found in free State in fruits and in some parts of the plants. It contains an aldehyde group and a six carbon atom.

The chemical formula is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$.
Molar mass is the arithmetic sum of atomic mass of elements in the molecule.
Molar mass = $\dfrac{Molecular\text{ }weight\text{ }in\text{ }gram}{1\text{ }mole}$
The mass of a particular atom of an element which is expressed in atomic mass units.
The atomic mass of carbon = 12
The atomic mass of hydrogen =1.008
The atomic weight of oxygen = 16
The molar mass of glucose = atomic mass of carbon$\times $6 + atomic mass of hydrogen$\times $12 + atomic mass of oxygen $\times $ 6
Any number divided by 1 gives the same number.
= 12 $\times $ 6 + 1.008 $\times $12 + 16 $\times $ 6
= 180.096g
So, from the above discussion we can conclude that option A is the correct answer.
Note:
It is very important to be careful while calculating. It is better to remember the atomic mass of elements in periodic tables. If we know the atomic mass of the elements and formula to calculate in hand, solving this question takes less time.180g means there is 180g per mole as the definition molar mass is measured in grams per mole. As we know that any number divided by 1 gives the same number.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




