
What is the molecular formula for trichloroisocyanuric acid?
Answer
516.6k+ views
Hint :The chemical symbols for the constituent elements are accompanied by numeric subscripts defining the number of atoms of each element found in the molecule in a molecular formula.
Complete Step By Step Answer:
Trichloroisocyanuric acid is an organic compound that is made up of three dichloroisocyanuric acids. There are $12$ atoms in the TRICHLOROISOCYANURIC ACID molecule (s). There are three carbon atoms, three nitrogen atoms, three oxygen atoms, and three chlorine atoms in this compound (s).
The molecular formula of trichloroisocyanuric acid is \[{C_3}C{l_3}{N_3}{O_3}\]. In this compound, three chlorine atoms are bonded to the isocyanate group.
By observing the molecular formula, the molecular structure of trichloroisocyanuric acid is given below –
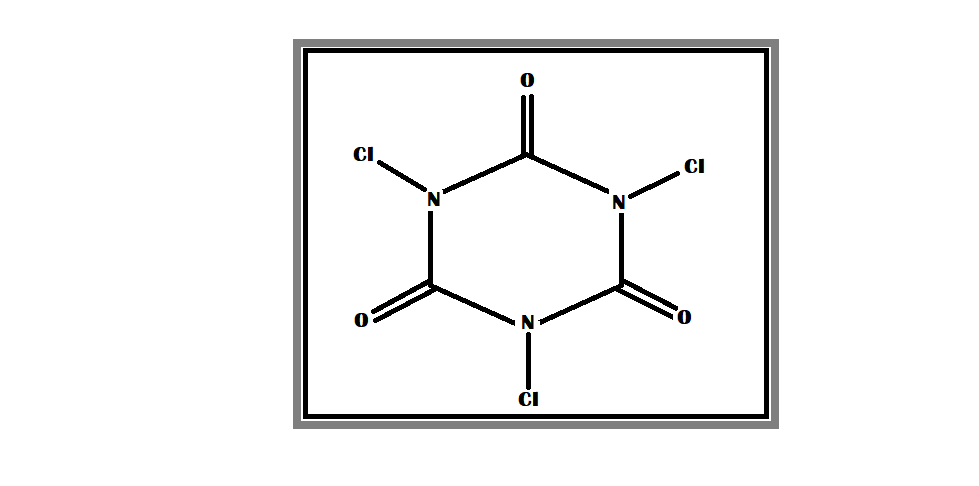
Trichloroisocyanuric acid's chemical formula is based on the molecular formula, which indicates the numbers of each type of atom in a molecule without structural detail, as opposed to the empirical formula, which gives numerical proportions of atoms of each type.
Stoichiometry in chemical equations, or the measurement of relative quantities of reactants and products in chemical reactions, is based on the chemical formula above. In a chemical reaction, the quantity of each element in the chemical formula does not change, according to the law of conservation of mass. As a result, based on the chemical formula, each side of the chemical equation must represent the same quantity of each product.
Note :
The compound is primarily used as a disinfectant, algicide, and bactericide in swimming pools and dyestuffs, and as a bleaching agent in the textile industry. It's commonly used in civil sanitation for pools and spas, preventing and curing diseases in livestock and fisheries, preserving fruits and vegetables.
Complete Step By Step Answer:
Trichloroisocyanuric acid is an organic compound that is made up of three dichloroisocyanuric acids. There are $12$ atoms in the TRICHLOROISOCYANURIC ACID molecule (s). There are three carbon atoms, three nitrogen atoms, three oxygen atoms, and three chlorine atoms in this compound (s).
The molecular formula of trichloroisocyanuric acid is \[{C_3}C{l_3}{N_3}{O_3}\]. In this compound, three chlorine atoms are bonded to the isocyanate group.
By observing the molecular formula, the molecular structure of trichloroisocyanuric acid is given below –

Trichloroisocyanuric acid's chemical formula is based on the molecular formula, which indicates the numbers of each type of atom in a molecule without structural detail, as opposed to the empirical formula, which gives numerical proportions of atoms of each type.
Stoichiometry in chemical equations, or the measurement of relative quantities of reactants and products in chemical reactions, is based on the chemical formula above. In a chemical reaction, the quantity of each element in the chemical formula does not change, according to the law of conservation of mass. As a result, based on the chemical formula, each side of the chemical equation must represent the same quantity of each product.
Note :
The compound is primarily used as a disinfectant, algicide, and bactericide in swimming pools and dyestuffs, and as a bleaching agent in the textile industry. It's commonly used in civil sanitation for pools and spas, preventing and curing diseases in livestock and fisheries, preserving fruits and vegetables.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




