
Molecular formula of benzoic acid is:
A.) \[{{C}_{6}}{{H}_{5}}COOH\]
B.) \[{{C}_{6}}{{H}_{6}}COOH\]
C.) \[{{C}_{5}}{{H}_{6}}COOH\]
D.) None
Answer
604.2k+ views
Hint: We know that benzoic acid is an acid and it is an organic compound. From the benzo word we can understand that it is a compound of benzene. And from the acid word we can understand that it has \[{{H}^{+}}\] ions.
Step by step solution:
Benzoic acid is an acid and can release \[{{H}^{+}}\] ion means hydrogen attached with a more electronegative element or group. Benzoic acid has six carbons, five hydrogens and one carboxylic acid group with it. So the chemical formula of benzoic acid is: \[{{C}_{6}}{{H}_{5}}COOH\]
And its chemical structure is:
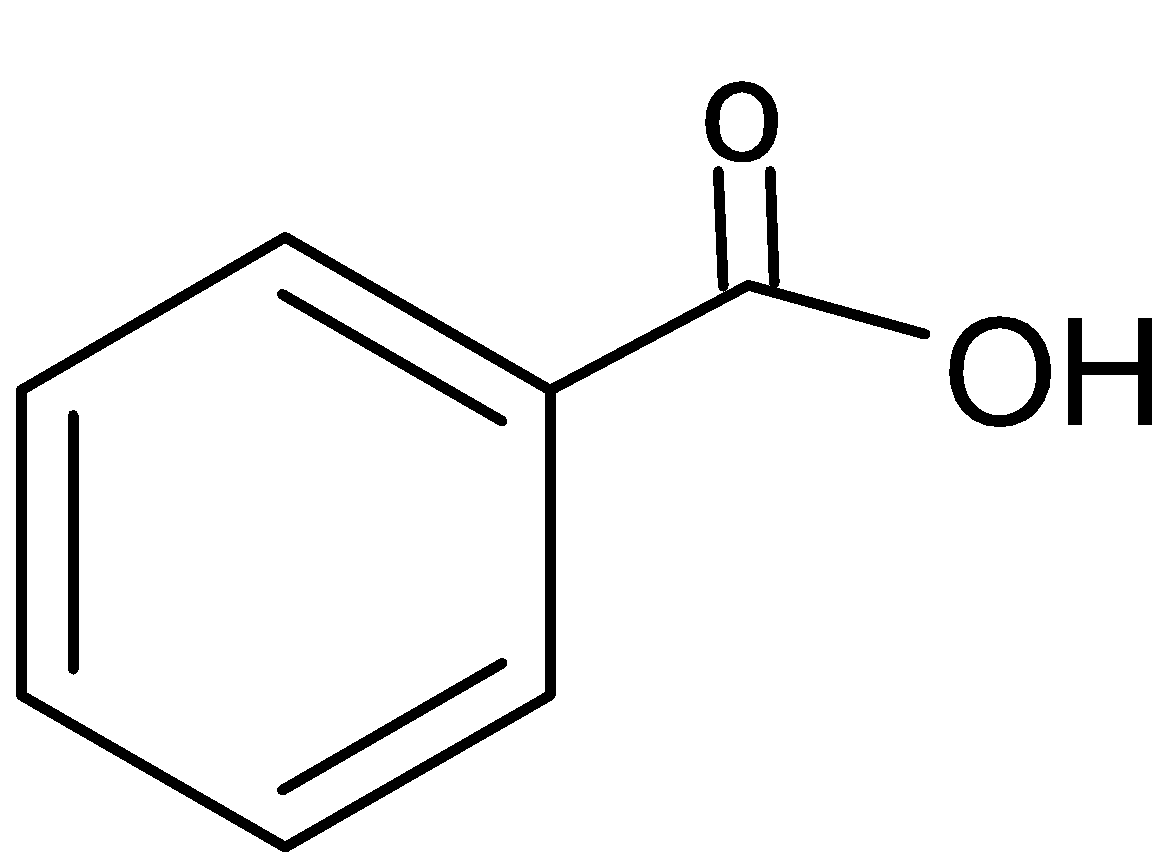 It is classified as an aromatic carboxylic acid. It’s aromatic due to the presence of a benzene ring in its chemical structure. And it’s a carboxylic acid due to the presence of carboxyl group (\[-COOH\]) in its structure. This acid is most commonly found in industrial settings to manufacture a wide variety of products such as perfumes, dyes etc.
It is classified as an aromatic carboxylic acid. It’s aromatic due to the presence of a benzene ring in its chemical structure. And it’s a carboxylic acid due to the presence of carboxyl group (\[-COOH\]) in its structure. This acid is most commonly found in industrial settings to manufacture a wide variety of products such as perfumes, dyes etc.
So, from the above explanation we can say that option “A” is the correct answer.
Option “B” is incorrect because it has extra hydrogen in it which doesn’t fulfill the valency rule of carbon or benzene.
Option “C” is incorrect because it is a 5 membered ring which is not a benzene ring.
Note: Here “ic” suffix is for carboxylic acid and benzo is for benzene. So, you should be careful while taking the acidic group. This Compound neutralizes the basic medium, which tells that it is an acid. It is also known as benzenecarboxylic acid.
Step by step solution:
Benzoic acid is an acid and can release \[{{H}^{+}}\] ion means hydrogen attached with a more electronegative element or group. Benzoic acid has six carbons, five hydrogens and one carboxylic acid group with it. So the chemical formula of benzoic acid is: \[{{C}_{6}}{{H}_{5}}COOH\]
And its chemical structure is:

So, from the above explanation we can say that option “A” is the correct answer.
Option “B” is incorrect because it has extra hydrogen in it which doesn’t fulfill the valency rule of carbon or benzene.
Option “C” is incorrect because it is a 5 membered ring which is not a benzene ring.
Note: Here “ic” suffix is for carboxylic acid and benzo is for benzene. So, you should be careful while taking the acidic group. This Compound neutralizes the basic medium, which tells that it is an acid. It is also known as benzenecarboxylic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




