
How many molecules of acetylene are required to form benzene?
A. $2$
B. $3$
C. $4$
D. $5$
Answer
532.2k+ views
Hint: The following reaction Propene reacts with \[HBr\] in presence of peroxide follows anti markovnikov rule. Anti markovnikov addition reaction is a reaction that takes place when an electrophile hydrogen halide reacts with alkene and alkyne, hydrogen of hydrogen halide will become bonded to the carbon that has least number of hydrogen atoms in an alkyne and alkene. This reaction uses peroxide as a catalyst.
Complete step by step answer:
Acetylene is a simplest alkyne with chemical formula ${{C}_{2}}{{H}_{2}}$ Chemical formula of benzene is ${{C}_{6}}{{H}_{6}}.$ so, if we take three molecules of ethyne or acetylene and react under further conditions we get the benzene.
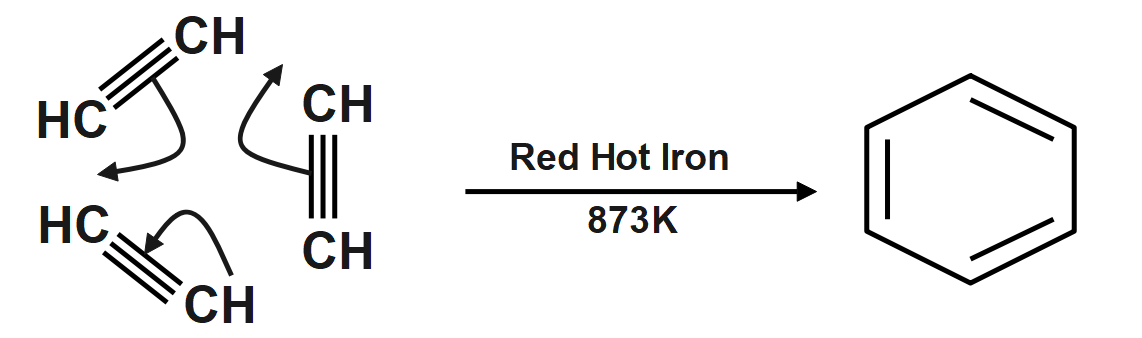
However, each carbon atom forms different bonds (single and double) with two carbon atoms which are again forming two dissimilar bonds with other carbon atoms. Therefore, the hybridisation of carbon on benzene is $s{{p}^{2}}$. Therefore, polymerization of acetylene to benzene changes hybridisation from $sp$ to $s{{p}^{2}}$ .
Formation of benzene from acetylene is a cyclic polymerization reaction, which is an intermolecular reaction and it is carried out at a very high temperature of $873K$We should not be confused by the two dissimilar bonds formed by benzene. Taking any of the six carbon atoms into consideration, we will get a carbon-carbon double bond.
So, the correct answer is Option B.
Note: Note that alkenes considered being in the group of unsaturated hydrocarbons where alkene must contain one double bond. In the presence of peroxide, the regioselectivity for the addition reaction of other electrophiles is not altered. This reaction mechanism has three steps that are initiation, propagation and termination.
Complete step by step answer:
Acetylene is a simplest alkyne with chemical formula ${{C}_{2}}{{H}_{2}}$ Chemical formula of benzene is ${{C}_{6}}{{H}_{6}}.$ so, if we take three molecules of ethyne or acetylene and react under further conditions we get the benzene.

However, each carbon atom forms different bonds (single and double) with two carbon atoms which are again forming two dissimilar bonds with other carbon atoms. Therefore, the hybridisation of carbon on benzene is $s{{p}^{2}}$. Therefore, polymerization of acetylene to benzene changes hybridisation from $sp$ to $s{{p}^{2}}$ .
Formation of benzene from acetylene is a cyclic polymerization reaction, which is an intermolecular reaction and it is carried out at a very high temperature of $873K$We should not be confused by the two dissimilar bonds formed by benzene. Taking any of the six carbon atoms into consideration, we will get a carbon-carbon double bond.
So, the correct answer is Option B.
Note: Note that alkenes considered being in the group of unsaturated hydrocarbons where alkene must contain one double bond. In the presence of peroxide, the regioselectivity for the addition reaction of other electrophiles is not altered. This reaction mechanism has three steps that are initiation, propagation and termination.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




