
Mononitration of phenyl methanoate.
Answer
526.5k+ views
Hint: Formate whose IUPAC name is methanoate is the anion derived from formic acid. Its formula is chloroformate, $C{H_3}OCOCl$. The combined influence of —$OH$ and —$C{H_3}$ groups determines the position of the incoming group.
Complete answer:
Phenyl methanoate is a compound having very less shelf- life. So, it is very unstable. Its formula is ${C_6}{H_5}OCOH$.
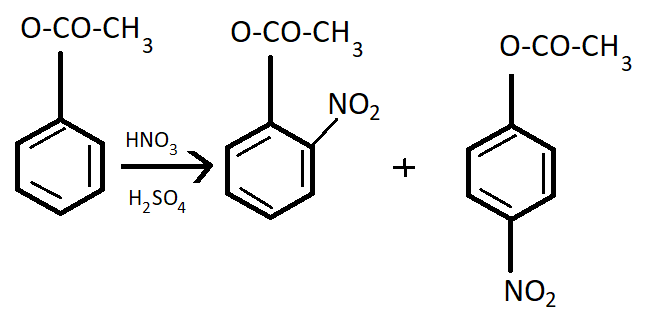
It goes to the para position because the Benzene ring will be unstable if the $N{O_2}$group is attached anywhere else. It is an electron donating group and also an unstable compound at Meta or Ortho positions.
This process occurs in the presence of $HN{O_3}$ and ${H_2}S{O_4}$which is the nitrating mixture.
Note: Phenyl methanoate is a compound having very less life and hence it is very unstable. Its nitration is not feasible to carry out. But still if nitration is carried out then it will give a meta product. In such types of problems, the properties of compounds are very important.
Complete answer:
Phenyl methanoate is a compound having very less shelf- life. So, it is very unstable. Its formula is ${C_6}{H_5}OCOH$.

It goes to the para position because the Benzene ring will be unstable if the $N{O_2}$group is attached anywhere else. It is an electron donating group and also an unstable compound at Meta or Ortho positions.
This process occurs in the presence of $HN{O_3}$ and ${H_2}S{O_4}$which is the nitrating mixture.
Note: Phenyl methanoate is a compound having very less life and hence it is very unstable. Its nitration is not feasible to carry out. But still if nitration is carried out then it will give a meta product. In such types of problems, the properties of compounds are very important.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




