
Mutarotation involves:
(A) Racemisation
(B) Diastereomer Isation
(C) Optical resolution
(D) Conformational inversion
Answer
582.3k+ views
Hint: Mutarotation is the change in optical relation observed when pure $\alpha - $or $\beta - $anomers are dissolved in water.
It is possible when $\alpha - $or $\beta - $anomers can interconvert.
Step by step answer: Let us discuss the terms given in question one by one:
Race misation: This is a process in which optically active compounds convert into an equal mixture of enantiomers with zero optical activity.
The mixture is also called racemic mixture. The mixture contains dextro- and laevo- rotatory isomers in equal quantity.
Siaslereomerisation: Let us explain thus with the following example.
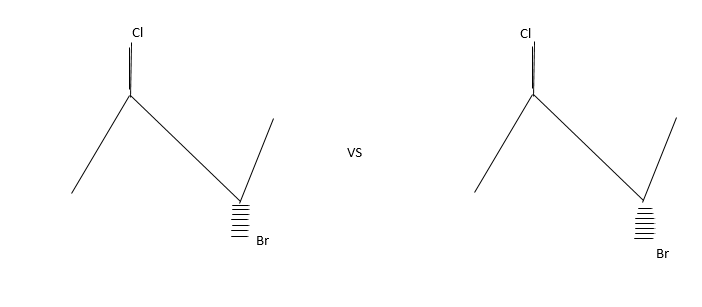
The compound contains both Bromine and chlorine atoms but they differed in the way they occupied three dimension dace.
These isomers are present in equilibrium and interconvertible.
Optical resolution: Optical resolution is a physical resolution at which an imaging device can capture an image.
Conformational inversion: Saturated cyclic six membered heterocycles i.e., cyclohexane can adopt one or more conformations that are free of torsion or bond angle strain.
Mutarotation involves Diastereomer Isation.
Therefore, from the above explanation the correct option is (B) Diastereomer Isation.
Note: Mutarotation is the change in optical rotation because of change in equilibrium between two anomers. The optical rotation of solution depends on the optical rotation of each anomer and their ratio in the solution. The $\alpha $and $\beta $anomers are diastereomers of each other and usually have different specific rotations.
It is possible when $\alpha - $or $\beta - $anomers can interconvert.
Step by step answer: Let us discuss the terms given in question one by one:
Race misation: This is a process in which optically active compounds convert into an equal mixture of enantiomers with zero optical activity.
The mixture is also called racemic mixture. The mixture contains dextro- and laevo- rotatory isomers in equal quantity.
Siaslereomerisation: Let us explain thus with the following example.

The compound contains both Bromine and chlorine atoms but they differed in the way they occupied three dimension dace.
These isomers are present in equilibrium and interconvertible.
Optical resolution: Optical resolution is a physical resolution at which an imaging device can capture an image.
Conformational inversion: Saturated cyclic six membered heterocycles i.e., cyclohexane can adopt one or more conformations that are free of torsion or bond angle strain.

Mutarotation involves Diastereomer Isation.
Therefore, from the above explanation the correct option is (B) Diastereomer Isation.
Note: Mutarotation is the change in optical rotation because of change in equilibrium between two anomers. The optical rotation of solution depends on the optical rotation of each anomer and their ratio in the solution. The $\alpha $and $\beta $anomers are diastereomers of each other and usually have different specific rotations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




