
${{\text{N}}_{\text{2}}}\text{CO}$ has three possible structures:
$\text{ONCN}$ (nitrosyl cyanide), $\text{ONNC}$ (nitrosyl isocyanide), $\text{NOCN}$ (iso nitrosyl cyanide)
Which of the following structures has the lowest potential energy?
A.$\text{ONCN}$
B.$\text{ONNC}$
C.$\text{NOCN}$
D.All have same energy
Answer
575.7k+ views
Hint:To solve this question, knowledge on the bond-formation energies between the different bonds is required. The triple bond between the nitrogen atoms in any structure has a high amount of energy because huge energy is required to break the triple bond and along with that, it is equally shared between two atoms.
Complete step by step answer:
In the above question, all the ligands are isomers with the molecular formula ${{\text{N}}_{\text{2}}}\text{CO}$. Given below are the different structures of the isomers of ${{\text{N}}_{\text{2}}}\text{CO}$.
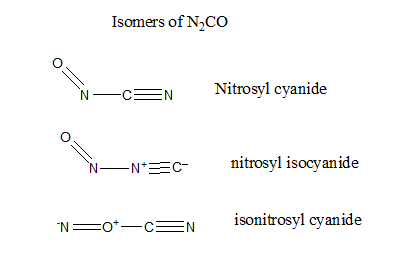
From the above figure it can be seen that nitrosyl cyanide has the most stable structure among all the isomers because all the formal charges are satisfied here. Also the triple bond between carbon and nitrogen atoms without any charges on the atoms makes it stable.
On the other hand in nitrosyl isocyanide, there is a positive charge on the nitrogen atom and a negative charge on the adjacent carbon atom, making it unstable similarly in iso nitryl cyanide, there is positive charge on the oxygen atom making the structure unstable.
Hence option A is correct.
Note:
Energy is released when bond is formed and required when bond is broken. This energy is stored in the form of potential energy in the bonds and is called Bond Formation Energy. The higher the number of bonds, the higher is the amount of energy required to break the bond.
Hence bond strength is in the following order: triple bond > double bond > single bond.
Complete step by step answer:
In the above question, all the ligands are isomers with the molecular formula ${{\text{N}}_{\text{2}}}\text{CO}$. Given below are the different structures of the isomers of ${{\text{N}}_{\text{2}}}\text{CO}$.

From the above figure it can be seen that nitrosyl cyanide has the most stable structure among all the isomers because all the formal charges are satisfied here. Also the triple bond between carbon and nitrogen atoms without any charges on the atoms makes it stable.
On the other hand in nitrosyl isocyanide, there is a positive charge on the nitrogen atom and a negative charge on the adjacent carbon atom, making it unstable similarly in iso nitryl cyanide, there is positive charge on the oxygen atom making the structure unstable.
Hence option A is correct.
Note:
Energy is released when bond is formed and required when bond is broken. This energy is stored in the form of potential energy in the bonds and is called Bond Formation Energy. The higher the number of bonds, the higher is the amount of energy required to break the bond.
Hence bond strength is in the following order: triple bond > double bond > single bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




