
What is the name of red azo dye?
(A) $\beta $-naphthyl azo benzene
(B) p-hydroxy azo benzene
(C) p-amino azo benzene
(D) p-N,N-dimethyl amino azo benzene
Answer
583.8k+ views
Hint: Azo dyes are mainly formed by the reaction between an aromatic compound and the intermediate formed during Sandmeyer's reaction. Now try to identify the different types of dyes that can be formed with this reaction. With this, you can figure out the name of the azo dye that imparts red colour to the solution.
Complete step by step solution:
Azo compounds are compounds consisting of the functional group diazinyl. The functional group is mentioned below:
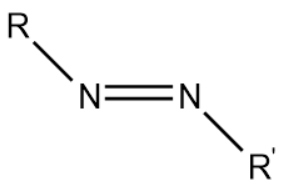
Where,
R and R' are alkyl or aryl groups
Stable azo compounds usually contain two aryl groups.
Azo dyes are organic compounds bearing the diazene functional group as defined by the International Union of Pure and Applied Chemistry(IUPAC). Azo dyes are widely used to treat textiles, leather articles and few foods as well.
One characteristic feature of azo dyes is their unique colour. They impart colour to the solution due to the alternating single and double bonds, known as a conjugated system. One such dye is the red dye.
The IUPAC name for red azo dye is p-N, N-dimethyl amino azo benzene. The structure for the compound is given below:
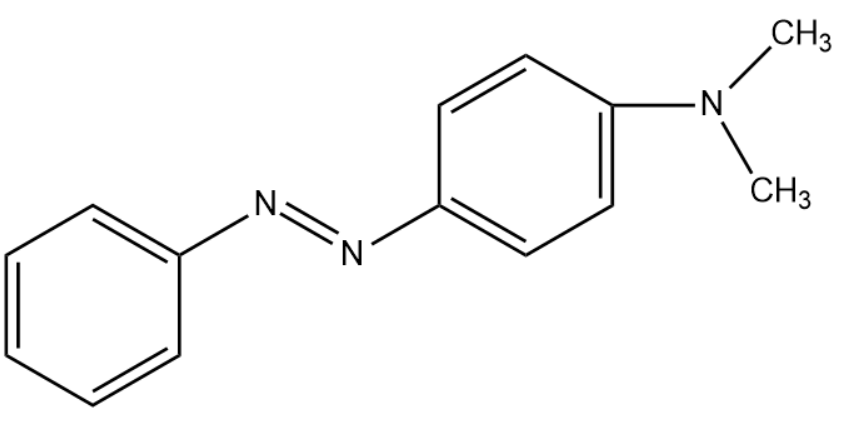
Note: Although many azo pigments are non-toxic, some compounds such as dinitroaniline orange, ortho-nitroaniline orange are found to be mutagenic. Along with that several case studies have reported azo pigments with basal cell carcinoma. Due to this, the European Union has prohibited the manufacture and sale of azo pigments since September 2003.
Complete step by step solution:
Azo compounds are compounds consisting of the functional group diazinyl. The functional group is mentioned below:

Where,
R and R' are alkyl or aryl groups
Stable azo compounds usually contain two aryl groups.
Azo dyes are organic compounds bearing the diazene functional group as defined by the International Union of Pure and Applied Chemistry(IUPAC). Azo dyes are widely used to treat textiles, leather articles and few foods as well.
One characteristic feature of azo dyes is their unique colour. They impart colour to the solution due to the alternating single and double bonds, known as a conjugated system. One such dye is the red dye.
The IUPAC name for red azo dye is p-N, N-dimethyl amino azo benzene. The structure for the compound is given below:

Note: Although many azo pigments are non-toxic, some compounds such as dinitroaniline orange, ortho-nitroaniline orange are found to be mutagenic. Along with that several case studies have reported azo pigments with basal cell carcinoma. Due to this, the European Union has prohibited the manufacture and sale of azo pigments since September 2003.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




