
Name the method used to refine copper metal.
A.Liquidation
B.Distillation
C.Zone refining
D.Electrolytic method
Answer
585k+ views
Hint: We can say that in the process of electrorefining, impurities such as iron, zinc and nickel get dissolved in the solution whereas gold, silver and platinum are deposited as anode mud under the anode and 100% copper is derived from crude copper.
Complete step by step answer:
We have to know in metallurgy, the process of refining plays an important role. Any metal extracted from its ore is impure and the impure metal that is extracted is known as crude metal.
We can obtain the purest form of copper by electrolytic method. Anode is impure copper metal, and cathode is the pure copper metal. We can use the acidic solution of copper sulfate as the electrolytic solution.
When we pass electric current, impure metal from anode liberates metal ions into the solution and from the solution, metal is obtained from metal ions at the cathode. The waster under the anode is called as anode mud. In the electrolytic refining of copper, the anode mud comprises zinc, silver, and gold etc.
The chemical reactions that take place in the anode and cathode are written as,
At anode, $Cu\,\left( {impure} \right)\xrightarrow{{}}C{u^{2 + }} + 2{e^ - }$
At cathode, $C{u^{2 + }} + 2{e^ - }\xrightarrow{{}}Cu\,\left( {pure} \right)$
We can draw the electro refining process of copper as,
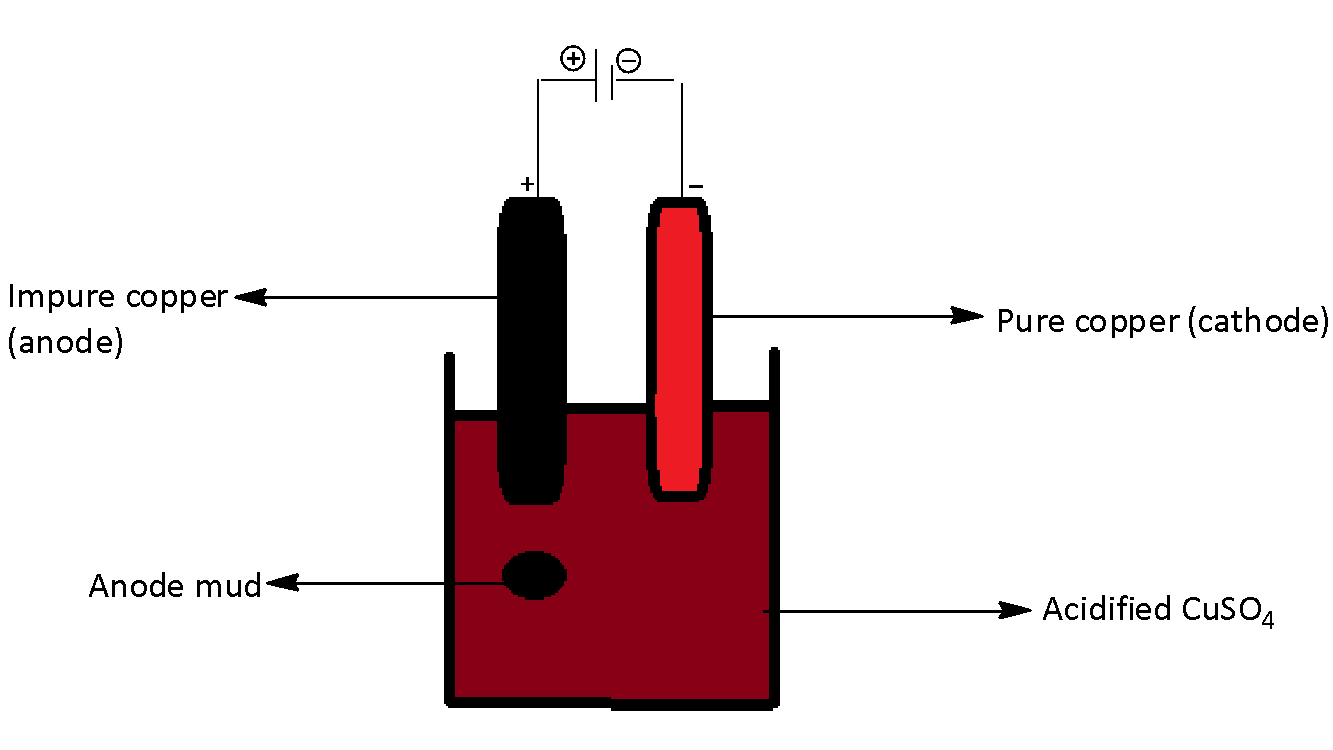
Therefore, Option (D) is correct.
Note:
We also know details about Mond’s process is the name for refining of nickel by vapour phase refining. The refining of zirconium is done by the Van Arkel method. We can obtain highly purified metals in the process of refining as it removes impurities. There are several methods to refine a metal based on properties of impurities and on the properties of metals. Some of the methods involved are,
Distillation
Liquation
Zone refining
Chromatography
Vapour phase refining
The process of refining depends on the metal’s nature and impurities's nature.
Complete step by step answer:
We have to know in metallurgy, the process of refining plays an important role. Any metal extracted from its ore is impure and the impure metal that is extracted is known as crude metal.
We can obtain the purest form of copper by electrolytic method. Anode is impure copper metal, and cathode is the pure copper metal. We can use the acidic solution of copper sulfate as the electrolytic solution.
When we pass electric current, impure metal from anode liberates metal ions into the solution and from the solution, metal is obtained from metal ions at the cathode. The waster under the anode is called as anode mud. In the electrolytic refining of copper, the anode mud comprises zinc, silver, and gold etc.
The chemical reactions that take place in the anode and cathode are written as,
At anode, $Cu\,\left( {impure} \right)\xrightarrow{{}}C{u^{2 + }} + 2{e^ - }$
At cathode, $C{u^{2 + }} + 2{e^ - }\xrightarrow{{}}Cu\,\left( {pure} \right)$
We can draw the electro refining process of copper as,

Therefore, Option (D) is correct.
Note:
We also know details about Mond’s process is the name for refining of nickel by vapour phase refining. The refining of zirconium is done by the Van Arkel method. We can obtain highly purified metals in the process of refining as it removes impurities. There are several methods to refine a metal based on properties of impurities and on the properties of metals. Some of the methods involved are,
Distillation
Liquation
Zone refining
Chromatography
Vapour phase refining
The process of refining depends on the metal’s nature and impurities's nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




