
Name the product obtained when nitrobenzene undergoes reduction with a metal and acid?
Answer
538.2k+ views
Hint:In order to know what will be the product formed when Nitrobenzene is reduced using Sn/HCl, we must first know what a reduction process is. Reduction is a chemical process which involves gain of the electron which will involve decrease in the oxidation number of the compound.
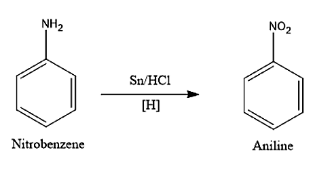
Complete step-by-step answer:Let us first understand what a reduction process is. Reduction is a chemical process which involves gain of the electron which will involve decrease in the oxidation number of the compound. In other words, we can say loss of the oxygen atom from the molecule and the gain of the hydrogen atom. Let us now move onto the question given. In this nitrobenzene will be reacting with the tin and the hydrochloric acid to give some product. Tin and Hydrochloric acid is a good reducing agent, it will remove the oxygen atom in the Nitrobenzene and will add the hydrogen atom to form the Aniline molecule. The reaction for the reduction of the Nitrobenzene is given below:
Note:There are also other reducing agents which can involve in the reduction process such as
- Lithium Aluminium hydride
- Boron hydride
- Sodium borohydride
- Nascent hydrogen
- Zinc amalgam
- potassium iodide
- Hydrogen peroxide
- Hydrazine
Complete step-by-step answer:Let us first understand what a reduction process is. Reduction is a chemical process which involves gain of the electron which will involve decrease in the oxidation number of the compound. In other words, we can say loss of the oxygen atom from the molecule and the gain of the hydrogen atom. Let us now move onto the question given. In this nitrobenzene will be reacting with the tin and the hydrochloric acid to give some product. Tin and Hydrochloric acid is a good reducing agent, it will remove the oxygen atom in the Nitrobenzene and will add the hydrogen atom to form the Aniline molecule. The reaction for the reduction of the Nitrobenzene is given below:

Note:There are also other reducing agents which can involve in the reduction process such as
- Lithium Aluminium hydride
- Boron hydride
- Sodium borohydride
- Nascent hydrogen
- Zinc amalgam
- potassium iodide
- Hydrogen peroxide
- Hydrazine
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




