
Name the reagents used in the following reactions:
1.Oxidation of primary alcohol to carboxylic acid
2.Oxidation of primary alcohol to aldehyde
3.Bromination of phenol to 2,4,6-tribromophenol
4.Benzyl alcohol to benzoic acid
5.Dehydration of propan-2-ol to propene
6.Butan-2-one to butan-2-ol
Answer
519.6k+ views
Hint: We have to know that alcohols are organic compounds that show hydroxyl groups present in them. We have to know that alcohols could be oxidized to carboxylic acids and aldehydes in the presence of oxidizing agents. We have to know that aldehydes and ketones could be reduced to primary alcohols and secondary alcohols respectively.
Complete answer:
1.Let us see the oxidation of primary alcohols to carboxylic acid
We can obtain carboxylic acid from oxidation of primary alcohols in the presence of strong oxidizing agents like acidified potassium permanganate. We can write the general equation is,
$R - OH\xrightarrow{{{K_2}C{r_2}{O_7}}}R - COOH$
We can use acidified potassium permanganate as a reagent for the oxidation of primary alcohol to carboxylic acid.
2.Let us see the oxidation of primary alcohol to aldehyde.
We can obtain aldehyde from oxidation of primary alcohols in the presence of mild oxidizing agents like pyridinium chlorochromate. We can write the general equation is,
$R - OH\xrightarrow[{C{H_2}C{l_2}}]{{PCC}}R - CHO$
We can use pyridinium chlorochromate in $C{H_2}C{l_2}$ as a reagent for the oxidation of primary alcohol to aldehyde.
3.Let us see the bromination of phenol to 2,4,6-tribromophenol.
We can obtain 2,4,6-tribromophenol by the bromination of phenol in aqueous bromine (or) bromine water. We can equation as,
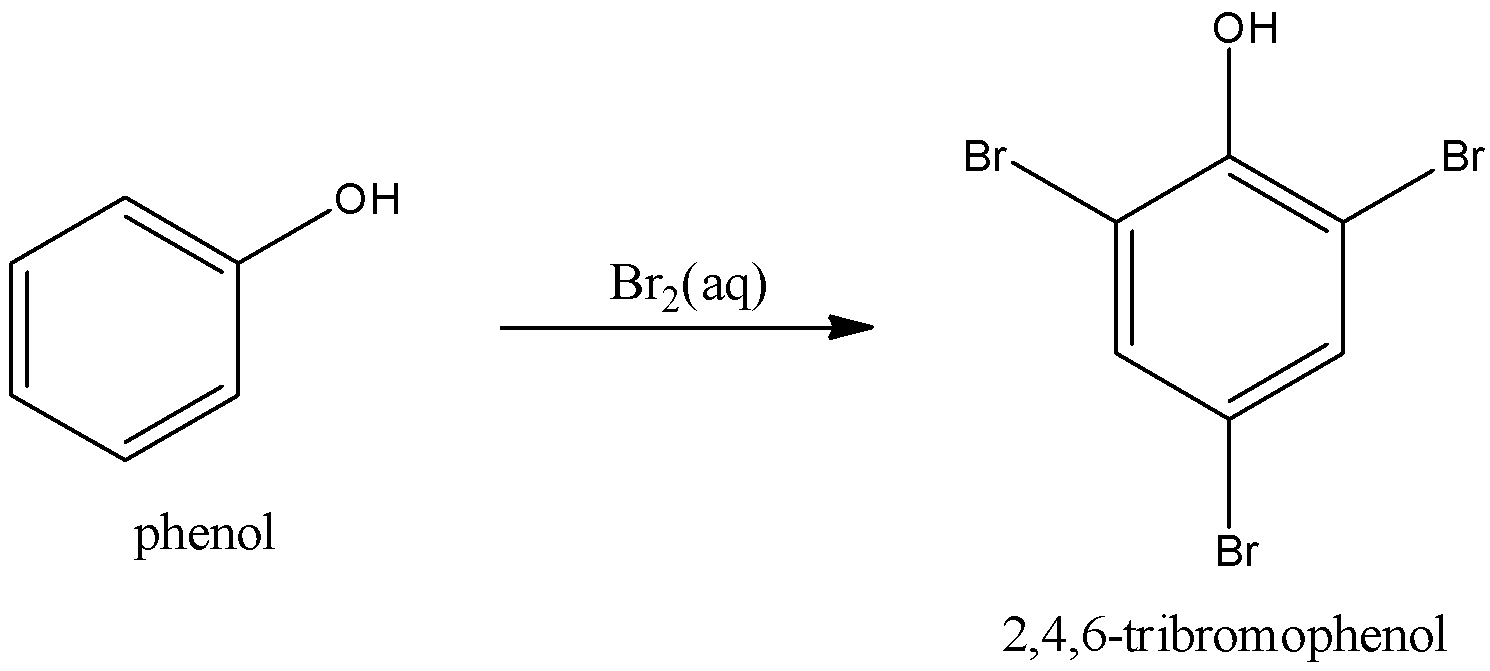 .
.
We can use aqueous bromine (or) bromine water as a reagent in the bromination of phenol to 2,4,6-tribromophenol.
4.Let us see now the conversion of benzyl alcohol to benzoic acid.
We can convert benzyl alcohol to benzoic acid using acidified $KMn{O_4}$ (or) alkaline $KMn{O_4}$. This is followed by hydrolysis with dilute sulfuric acid. We can write the chemical equation as,
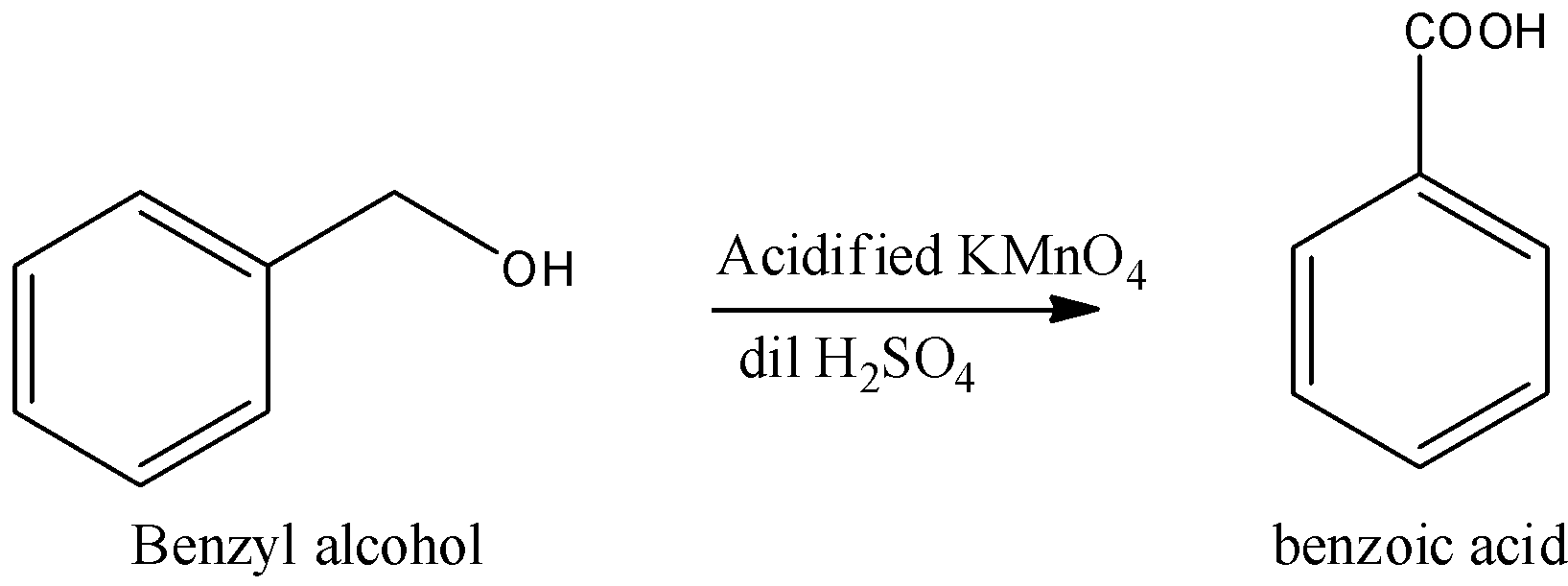
We can use acidified $KMn{O_4}$ (or) alkaline $KMn{O_4}$. This is followed by hydrolysis with dilute sulfuric acid as a reagent in the conversion of benzyl alcohol to benzoic acid.
5.Let us now see the dehydration reaction of propan-2-ol to propene.
We can use 85% phosphoric acid as a reagent for the dehydration reaction of propan-2-ol to propene. We can write the chemical equation as,
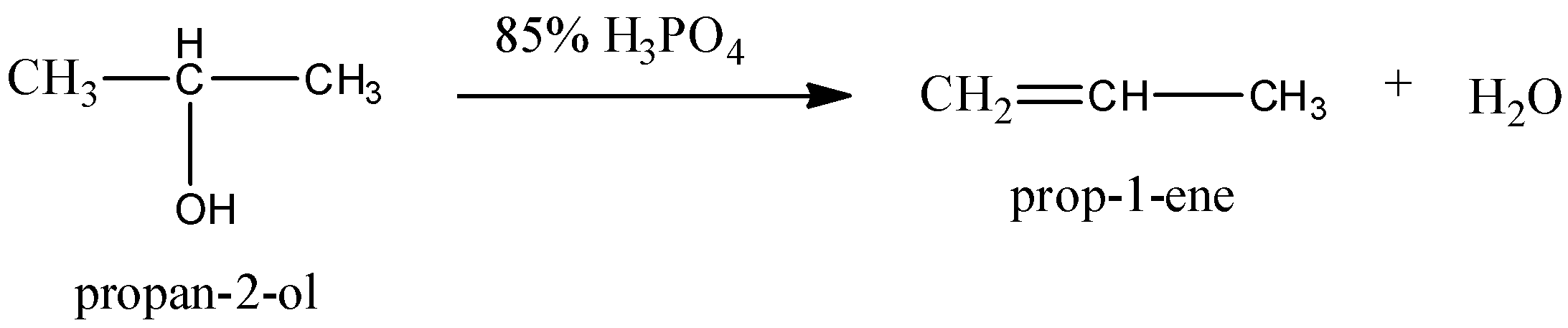
6.Let us now see the conversion of Butan-2-one to butan-2-ol. We know that butan-2-one is a ketone and butan-2-ol is a secondary alcohol. Ketone to secondary alcohol is a reduction reaction. The reduction reaction happens in the presence of reducing agents like sodium borohydride and Lithium aluminum hydride. We can write the chemical equation is,
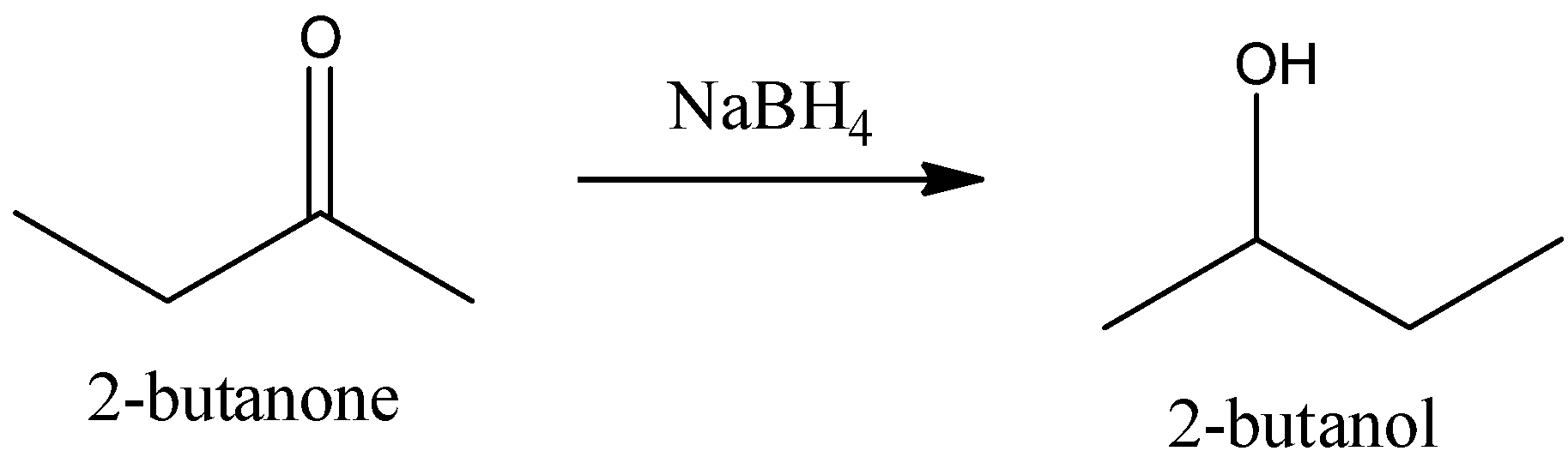
We can use sodium borohydride (or) lithium aluminum hydride as a reagent for the conversion of 2-butanone to 2-butanol.
Note:
We have to remember that stronger oxidizing agents convert primary alcohol to carboxylic acids directly. Milder oxidizing agents convert secondary alcohols to ketones and primary alcohols to aldehydes. Tertiary alcohols do not undergo any oxidation reaction with alcohols.
Complete answer:
1.Let us see the oxidation of primary alcohols to carboxylic acid
We can obtain carboxylic acid from oxidation of primary alcohols in the presence of strong oxidizing agents like acidified potassium permanganate. We can write the general equation is,
$R - OH\xrightarrow{{{K_2}C{r_2}{O_7}}}R - COOH$
We can use acidified potassium permanganate as a reagent for the oxidation of primary alcohol to carboxylic acid.
2.Let us see the oxidation of primary alcohol to aldehyde.
We can obtain aldehyde from oxidation of primary alcohols in the presence of mild oxidizing agents like pyridinium chlorochromate. We can write the general equation is,
$R - OH\xrightarrow[{C{H_2}C{l_2}}]{{PCC}}R - CHO$
We can use pyridinium chlorochromate in $C{H_2}C{l_2}$ as a reagent for the oxidation of primary alcohol to aldehyde.
3.Let us see the bromination of phenol to 2,4,6-tribromophenol.
We can obtain 2,4,6-tribromophenol by the bromination of phenol in aqueous bromine (or) bromine water. We can equation as,

We can use aqueous bromine (or) bromine water as a reagent in the bromination of phenol to 2,4,6-tribromophenol.
4.Let us see now the conversion of benzyl alcohol to benzoic acid.
We can convert benzyl alcohol to benzoic acid using acidified $KMn{O_4}$ (or) alkaline $KMn{O_4}$. This is followed by hydrolysis with dilute sulfuric acid. We can write the chemical equation as,

We can use acidified $KMn{O_4}$ (or) alkaline $KMn{O_4}$. This is followed by hydrolysis with dilute sulfuric acid as a reagent in the conversion of benzyl alcohol to benzoic acid.
5.Let us now see the dehydration reaction of propan-2-ol to propene.
We can use 85% phosphoric acid as a reagent for the dehydration reaction of propan-2-ol to propene. We can write the chemical equation as,

6.Let us now see the conversion of Butan-2-one to butan-2-ol. We know that butan-2-one is a ketone and butan-2-ol is a secondary alcohol. Ketone to secondary alcohol is a reduction reaction. The reduction reaction happens in the presence of reducing agents like sodium borohydride and Lithium aluminum hydride. We can write the chemical equation is,

We can use sodium borohydride (or) lithium aluminum hydride as a reagent for the conversion of 2-butanone to 2-butanol.
Note:
We have to remember that stronger oxidizing agents convert primary alcohol to carboxylic acids directly. Milder oxidizing agents convert secondary alcohols to ketones and primary alcohols to aldehydes. Tertiary alcohols do not undergo any oxidation reaction with alcohols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




