
What is the net change in activation energy for the catalyzed reaction shown in the diagram above?
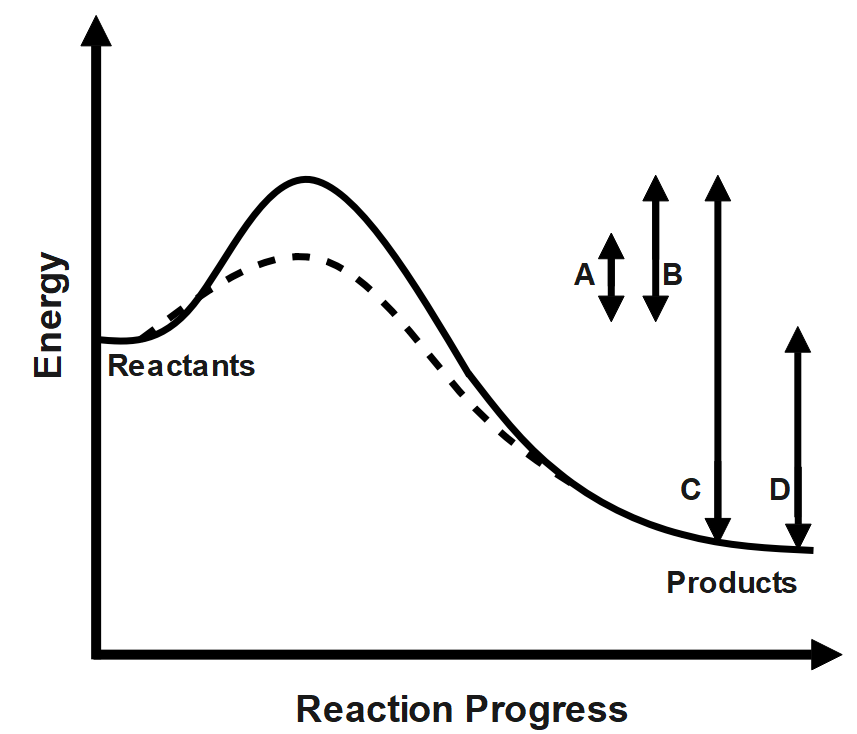
(A) A
(B) B
(C) B-A
(D) B-C
(E) A-C

Answer
502.2k+ views
Hint: We know that the activation energy of a chemical reaction is the amount of energy required to start a chemical reaction, activation energy is less for a spontaneous reaction and is high for a non-spontaneous reaction. The reaction given in the question has the forward and backward reaction energy as and respectively.
Complete answer:
As we can see, the graph that has been given to us is known as the activation energy graph. To get the meanings of the given variables X and Y, we must first learn to read the graph properly. To read the graph properly, we must first look at both the axes and try to understand the values that are being represented on these axes.
We can see that the y axis represents the potential energy of the reaction. Now the meaning of potential energy may differ slightly from the meaning that we have learnt in physics. In this context, the potential energy axis represents just the energy state of the reaction. The difference in this potential energy can thus calculate the difference in the energy states at any two points in the reaction. The x axis on the other hand represents the reaction coordinate. This axis basically marks the various states that the reactants and products go through during the course of the reaction. This axis is time independent.
Now, since the reaction is an endothermic one, it would consume energy during its course of action, this implies that for the endothermic reaction the forward energy will be higher. Lessening the activation energy faster will be the rate of a reaction. A catalyst can be used to reduce the activation energy. High activation energy can be understood as higher energy required to achieve a successful collision between the particles. A good example for this could be combustion. Even though combustion is an exothermic reaction, because of the high activation energy it requires heat. The particle gains enough energy from heat to overcome the barrier of activation energy. Thus, according to figure the original activation energy \[=B;\] New activation energy \[=A\] and new change in activation energy \[=B-A.\]
Therefore, the correct answer is option C.
Note:
Remember that the reactions which take more energy to break the bonds of the reactants are known as an endothermic reaction. In such reactions, the heat from the surroundings is absorbed as a result, the drop is the temperature occurs.
Complete answer:
As we can see, the graph that has been given to us is known as the activation energy graph. To get the meanings of the given variables X and Y, we must first learn to read the graph properly. To read the graph properly, we must first look at both the axes and try to understand the values that are being represented on these axes.
We can see that the y axis represents the potential energy of the reaction. Now the meaning of potential energy may differ slightly from the meaning that we have learnt in physics. In this context, the potential energy axis represents just the energy state of the reaction. The difference in this potential energy can thus calculate the difference in the energy states at any two points in the reaction. The x axis on the other hand represents the reaction coordinate. This axis basically marks the various states that the reactants and products go through during the course of the reaction. This axis is time independent.
Now, since the reaction is an endothermic one, it would consume energy during its course of action, this implies that for the endothermic reaction the forward energy will be higher. Lessening the activation energy faster will be the rate of a reaction. A catalyst can be used to reduce the activation energy. High activation energy can be understood as higher energy required to achieve a successful collision between the particles. A good example for this could be combustion. Even though combustion is an exothermic reaction, because of the high activation energy it requires heat. The particle gains enough energy from heat to overcome the barrier of activation energy. Thus, according to figure the original activation energy \[=B;\] New activation energy \[=A\] and new change in activation energy \[=B-A.\]
Therefore, the correct answer is option C.
Note:
Remember that the reactions which take more energy to break the bonds of the reactants are known as an endothermic reaction. In such reactions, the heat from the surroundings is absorbed as a result, the drop is the temperature occurs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




