
Nitrating mixture is obtained by mixing of $Conc.{\text{HN}}{{\text{O}}_3}{\text{ and Conc}}{\text{. }}{{\text{H}}_2}S{O_4}.$ role of ${H_2}S{O_4}$ in nitration is …………………….
This question has multiple correct options:
(A) To force $HN{O_3}$ to behave as a base
(B) To suppress the dissociation of $HN{O_3}$
(C) To produce $N{O^ + }_2$ ions.
(D) To remove the colour $N{O_2}$ produced during nitration.
Answer
594k+ views
Hint: Sulphuric acid is necessary as it helps in the generation of nitronium ion from nitric acid which is the actual nitrating agent. Nitronium ion is not easily produced when we use only nitric acid.
Complete answer:
Nitrating mixture is obtained by mixing of $Conc.{\text{ HN}}{{\text{O}}_3}{\text{ and Conc}}{\text{.}}{{\text{H}}_2}S{O_4}$
The role of ${H_2}S{O_4}$in nitrating is to produce nitronium ion by the protonation of nitric acid which causes the less water molecule and formation of nitronium ion.
In nitration of benzene
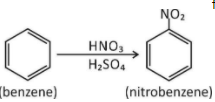
First step $ \to $ Generation of electrophile in nitration of benzene is to activate $HN{O_3}$with sulphuric acid to produce a stronger nucleophile, the nitronium ion.
Because the nitronium ion is a good electrophile, it is attacked by benzene to produce nitrobenzene.
Hence the correct option is A, B and C.
Note:In nitrating mixture, $N{O^ + }_2$ion is an electrophile. It is produced by transfer of proton from sulphuric acid to nitric acid. Hence $HN{O_3}$acts as a base. Nitration is used to add nitrogen to the benzene ring which can be used further in the substitution reaction. The nitro group acts as a ring deactivator. Nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term is applied to the different processes of forming nitrate esters between alcohols and nitric acid.
Complete answer:
Nitrating mixture is obtained by mixing of $Conc.{\text{ HN}}{{\text{O}}_3}{\text{ and Conc}}{\text{.}}{{\text{H}}_2}S{O_4}$
The role of ${H_2}S{O_4}$in nitrating is to produce nitronium ion by the protonation of nitric acid which causes the less water molecule and formation of nitronium ion.
In nitration of benzene

First step $ \to $ Generation of electrophile in nitration of benzene is to activate $HN{O_3}$with sulphuric acid to produce a stronger nucleophile, the nitronium ion.
Because the nitronium ion is a good electrophile, it is attacked by benzene to produce nitrobenzene.
Hence the correct option is A, B and C.
Note:In nitrating mixture, $N{O^ + }_2$ion is an electrophile. It is produced by transfer of proton from sulphuric acid to nitric acid. Hence $HN{O_3}$acts as a base. Nitration is used to add nitrogen to the benzene ring which can be used further in the substitution reaction. The nitro group acts as a ring deactivator. Nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term is applied to the different processes of forming nitrate esters between alcohols and nitric acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




