
Nitrobenzene is:
i.Steam Volatile
ii.Used as a solvent
iii.Aliphatic Compound
iv.Lighter than water
A.(i) and (ii) are correct
B.(iii) and (iv) are correct
C.(i) and (iii) are correct
D.(i) and (iv) are correct
Answer
512.4k+ views
Hint: For such kinds of questions, we need to know the structure of the given compound, then only we will be able to know whether the given compound is steam volatile or not, and many other things can also be known from the structure.
Complete answer:
Before finding out the answer to this question, let’s first understand what nitrobenzene is.
Nitrobenzene is known as an organic compound which having chemical formula of ${C_6}{H_5}N{O_2}$, that is, it is an aromatic compound which means it has nitro group $( - N{O_2})$ attached to a benzene ring $( - {C_6}{H_5})$. It is a yellow colored oil and smells like almond. Nitrobenzene is prepared by nitration of benzene, that is, benzene is made to react with nitric acid$( - HN{O_3})$ ad sulphuric acid $( - {H_2}S{O_4})$ which lead to the formation of nitrobenzene.
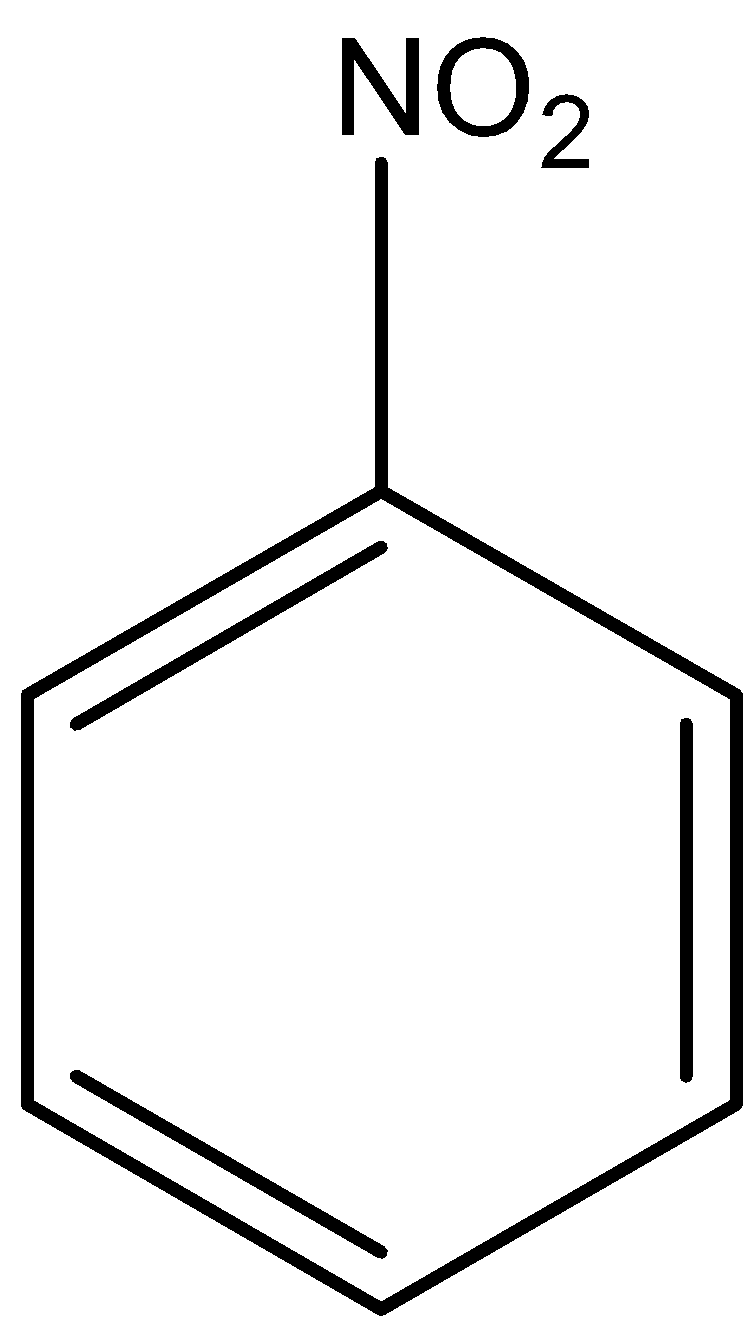
Now, let’s see what causes a compound to be steam volatile. So, the reason for this is hydrogen bonding, that is, an interaction which occurs between hydrogen and atoms such as oxygen, fluorine or nitrogen. So, as we know, nitrobenzene consists of nitrogen atoms in it and this nitrogen atom has the ability to form bonds with hydrogen atoms from steam. So, nitrobenzene is steam volatile.
Nitrobenzene is also used as a solvent due to the presence of an electron withdrawing group attached to the benzene, that is, nitro group.
Nitrobenzene is not an aliphatic compound which can be clearly seen from its structure. It is an aromatic compound having an aromatic ring in it. Therefore, option iii can be ruled out.
And nitrobenzene is not lighter than water. It is heavier than water.
Therefore, we can conclude that the correct option is Option A- (i) and (ii) are the correct options.
Note:
Nitrobenzene needs to be handled with precautions as it is very toxic in nature, it has the ability to get absorbed through skin. Long exposure to nitrobenzene can lead to several serious damages such as damage to our central nervous system, liver damage, impaired vision, etc.
Complete answer:
Before finding out the answer to this question, let’s first understand what nitrobenzene is.
Nitrobenzene is known as an organic compound which having chemical formula of ${C_6}{H_5}N{O_2}$, that is, it is an aromatic compound which means it has nitro group $( - N{O_2})$ attached to a benzene ring $( - {C_6}{H_5})$. It is a yellow colored oil and smells like almond. Nitrobenzene is prepared by nitration of benzene, that is, benzene is made to react with nitric acid$( - HN{O_3})$ ad sulphuric acid $( - {H_2}S{O_4})$ which lead to the formation of nitrobenzene.

Now, let’s see what causes a compound to be steam volatile. So, the reason for this is hydrogen bonding, that is, an interaction which occurs between hydrogen and atoms such as oxygen, fluorine or nitrogen. So, as we know, nitrobenzene consists of nitrogen atoms in it and this nitrogen atom has the ability to form bonds with hydrogen atoms from steam. So, nitrobenzene is steam volatile.
Nitrobenzene is also used as a solvent due to the presence of an electron withdrawing group attached to the benzene, that is, nitro group.
Nitrobenzene is not an aliphatic compound which can be clearly seen from its structure. It is an aromatic compound having an aromatic ring in it. Therefore, option iii can be ruled out.
And nitrobenzene is not lighter than water. It is heavier than water.
Therefore, we can conclude that the correct option is Option A- (i) and (ii) are the correct options.
Note:
Nitrobenzene needs to be handled with precautions as it is very toxic in nature, it has the ability to get absorbed through skin. Long exposure to nitrobenzene can lead to several serious damages such as damage to our central nervous system, liver damage, impaired vision, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




