
What is NOT true for aceto-phenone?
(A) It gives 2,4-DNP test
(B) It forms ${C_7}{H_6}{O_2}$ on drastic oxidation with $KMn{O_4},{H^ + }$
(C) It does not give Tollens or Fehling’s test
(D) It gives yellow ppt with NaOH, $C{l_2}$
Answer
588.9k+ views
Hint: Aceto-phenone has a ketone functional group and hence there are no hydrogens attached to the carbonyl carbon. Now identify which of the statements is false for ketones.
Complete step by step answer:
-First of all let us see the structure of aceto-phenone. It has a molecular formula of ${C_8}{H_8}O$ and the functional group present in it is a ketone group.
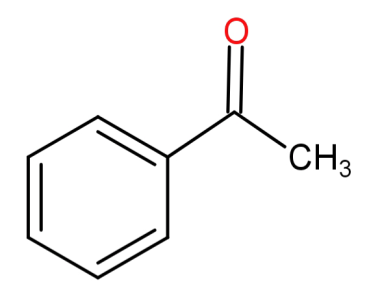
-Now we need to check each option to see which of these statements is not true for aceto-phenone.
-For (A) It gives 2,4-DNP test: 2,4 DNP reacts with both aldehydes and ketones. Here aceto-phenone will react with 2,4-Dinitrophenylhydrazine and forms its derivative called the 2,4-Dinitrophenylhydrazone. The reaction can be shown below:
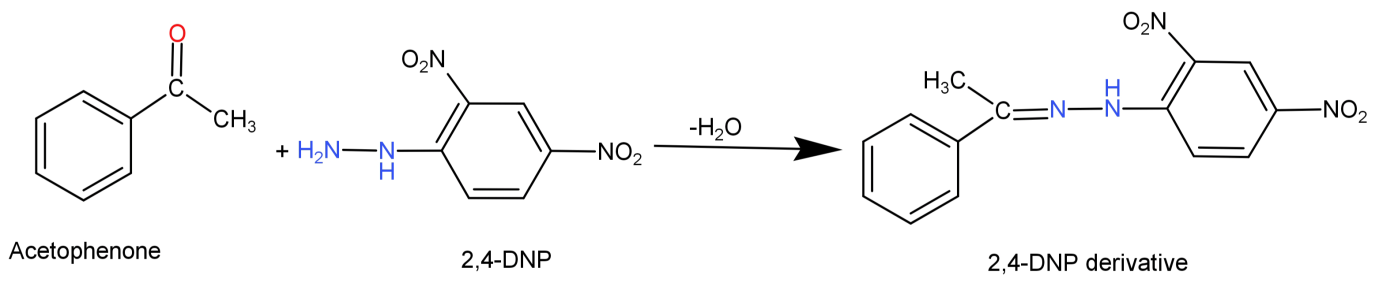
So, we can say that this statement is true.
-For (B) It forms ${C_7}{H_6}{O_2}$ on drastic oxidation with $KMn{O_4},{H^ + }$: When aceto-phenone undergoes drastic oxidation with $KMn{O_4},{H^ + }$ it leads to the breaking or rupture of C-C bond and thus forms benzoic acid which has a molecular formula of ${C_7}{H_6}{O_2}$. This reaction is shown below:
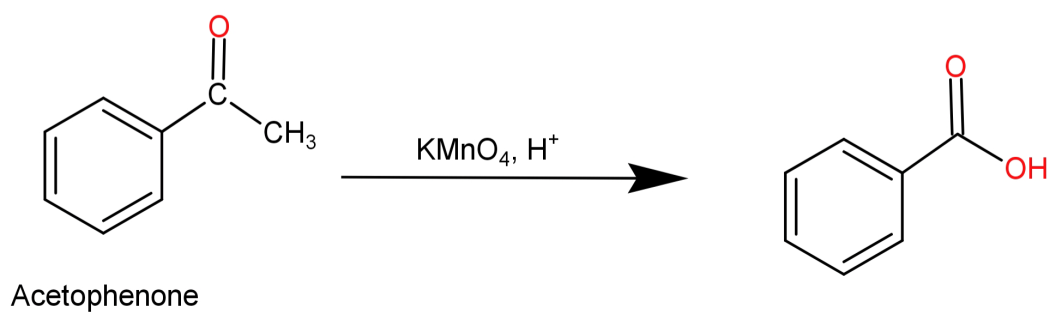
So, this statement is also true.
-For (C) It does not give Tollens or Fehling’s test: They give tests for aldehydes or say compounds which have a hydrogen atom attached to the carbonyl carbon. But here in aceto-phenone there is no such hydrogen atom which is attached to the carbonyl carbon (like in aldehydes) and so they do not give a test for aldehydes and ketones.So, this statement is also true.
-For (D) It gives yellow ppt with NaOH, $C{l_2}$: When aceto-phenone reacts with NaOH and $C{l_2}$, it leads to the formation of chloroform which is colourless. There is no formation of any yellow ppt. This reaction is shown below:
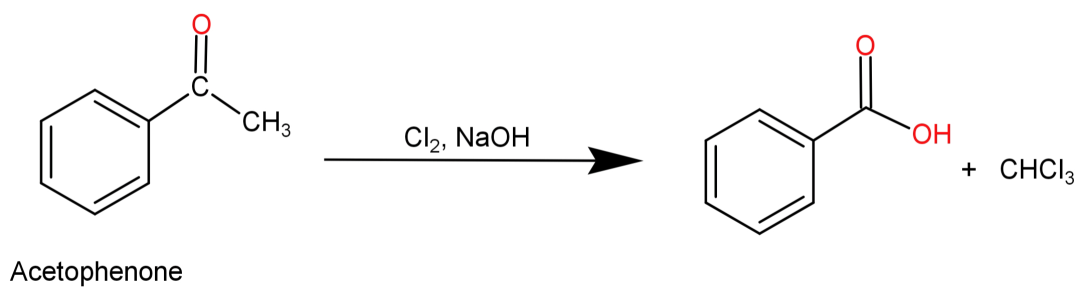
So, this statement is false. So, the correct answer is “Option D”.
Note: Aceto-phenone is the simplest aromatic ketone and has many pharmaceutical applications. It is used in the production of resins and key ingredients in fragrances resembling jasmine, cherry, almond, strawberry, etc.
Complete step by step answer:
-First of all let us see the structure of aceto-phenone. It has a molecular formula of ${C_8}{H_8}O$ and the functional group present in it is a ketone group.

-Now we need to check each option to see which of these statements is not true for aceto-phenone.
-For (A) It gives 2,4-DNP test: 2,4 DNP reacts with both aldehydes and ketones. Here aceto-phenone will react with 2,4-Dinitrophenylhydrazine and forms its derivative called the 2,4-Dinitrophenylhydrazone. The reaction can be shown below:

So, we can say that this statement is true.
-For (B) It forms ${C_7}{H_6}{O_2}$ on drastic oxidation with $KMn{O_4},{H^ + }$: When aceto-phenone undergoes drastic oxidation with $KMn{O_4},{H^ + }$ it leads to the breaking or rupture of C-C bond and thus forms benzoic acid which has a molecular formula of ${C_7}{H_6}{O_2}$. This reaction is shown below:

So, this statement is also true.
-For (C) It does not give Tollens or Fehling’s test: They give tests for aldehydes or say compounds which have a hydrogen atom attached to the carbonyl carbon. But here in aceto-phenone there is no such hydrogen atom which is attached to the carbonyl carbon (like in aldehydes) and so they do not give a test for aldehydes and ketones.So, this statement is also true.
-For (D) It gives yellow ppt with NaOH, $C{l_2}$: When aceto-phenone reacts with NaOH and $C{l_2}$, it leads to the formation of chloroform which is colourless. There is no formation of any yellow ppt. This reaction is shown below:

So, this statement is false. So, the correct answer is “Option D”.
Note: Aceto-phenone is the simplest aromatic ketone and has many pharmaceutical applications. It is used in the production of resins and key ingredients in fragrances resembling jasmine, cherry, almond, strawberry, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




