
Number of chiral atoms in Glucose and Fructose are:
(A).4 in each
(B).3 in each
(C).4 in glucose and 3 in fructose
(D).3 in glucose and 4 in fructose
Answer
591.6k+ views
Hint: Glucose and fructose are isomers, i.e. they have the same chemical formula but differ in connectivity.
Complete answer:
First of all, a chiral carbon is a carbon which has four different atoms or groups of atoms attached to it. The chiral atom in a molecule makes it optically active. Glucose and fructose are structural isomers. Their molecular formula is ${C_6}{H_{12}}{O_6}$. They both have different parent functional groups. The functional group in glucose is an aldehyde, while in fructose is a ketone.
The open chain structure of glucose is:
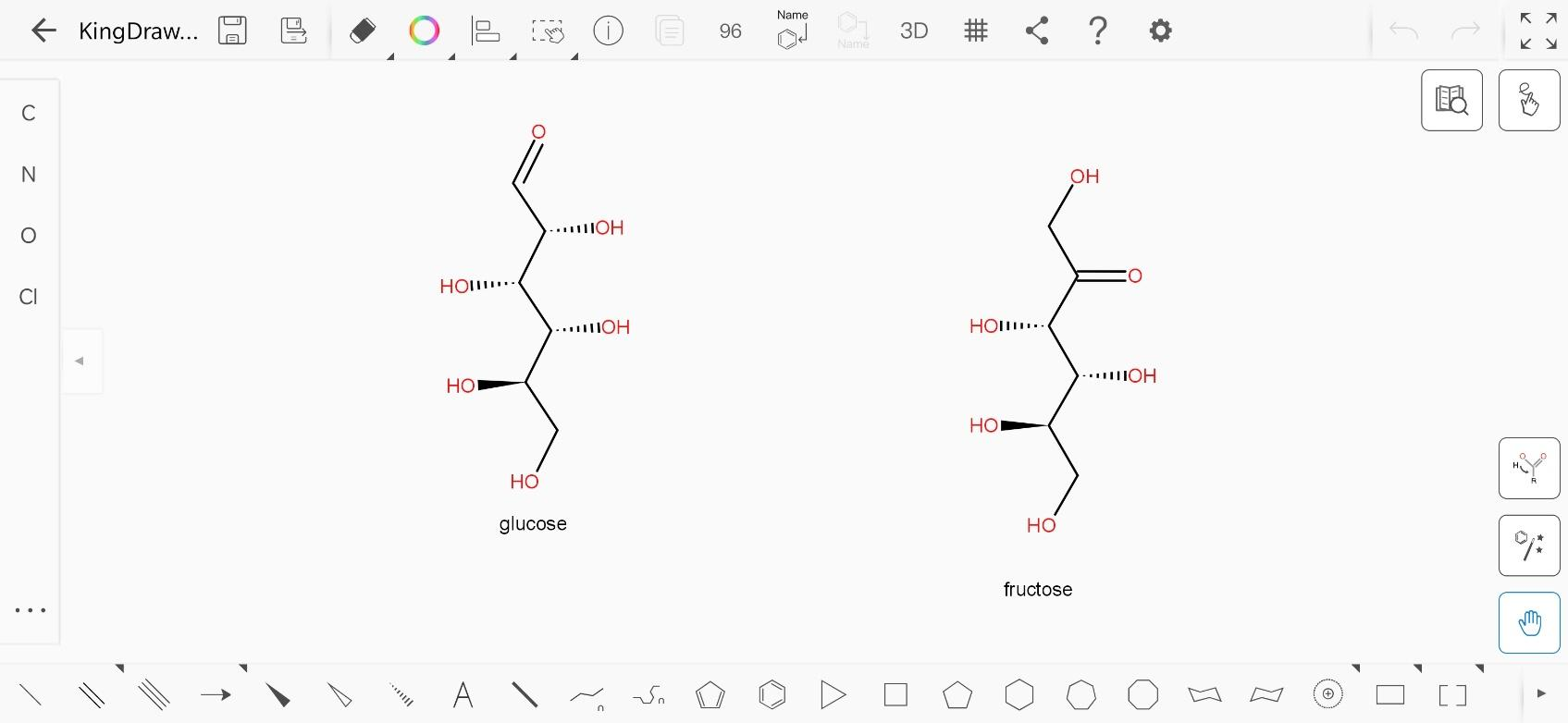
And the open chain structure of fructose is:

A chiral carbon is always an $s{p^3}$ carbon. If we number the carbon atoms from the top in each structure, the carbon numbered 2,3,4 and 5 are chiral in glucose as they have 4 different atoms or molecules attached to them. Similarly, carbon numbered 1,3,4,5 are chiral in fructose.
Hence, the number of chiral atoms in glucose and fructose are 4 each.
Additional information:
Generally, glucose and fructose don’t exist in this open chain form. For the sake of convenience, we imagine their open chained structures. But in reality, they exist in chain forms. Glucose is a six membered ring while fructose is a five membered ring.
Note:
A student might also consider $s{p^2}$ carbon to be chiral if it has all different atoms or molecules attached to it, but only $s{p^3}$ carbons can be classified as chiral or achiral.
Complete answer:
First of all, a chiral carbon is a carbon which has four different atoms or groups of atoms attached to it. The chiral atom in a molecule makes it optically active. Glucose and fructose are structural isomers. Their molecular formula is ${C_6}{H_{12}}{O_6}$. They both have different parent functional groups. The functional group in glucose is an aldehyde, while in fructose is a ketone.
The open chain structure of glucose is:

And the open chain structure of fructose is:

A chiral carbon is always an $s{p^3}$ carbon. If we number the carbon atoms from the top in each structure, the carbon numbered 2,3,4 and 5 are chiral in glucose as they have 4 different atoms or molecules attached to them. Similarly, carbon numbered 1,3,4,5 are chiral in fructose.
Hence, the number of chiral atoms in glucose and fructose are 4 each.
Additional information:
Generally, glucose and fructose don’t exist in this open chain form. For the sake of convenience, we imagine their open chained structures. But in reality, they exist in chain forms. Glucose is a six membered ring while fructose is a five membered ring.
Note:
A student might also consider $s{p^2}$ carbon to be chiral if it has all different atoms or molecules attached to it, but only $s{p^3}$ carbons can be classified as chiral or achiral.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




