
Number of \[dibromo\] derivatives produced when \[bromine\] is added to \[\left( {3S} \right) - 3 - Methyl{\text{ }}Cyclohexene\] in \[1,2 - dichloroethane\] are:
A.\[2\]
B.\[3\]
C.\[4\]
D.\[6\]
Answer
508.8k+ views
Hint: \[dibromo\] derivatives as the name suggests contains two \[bromine\] groups in the molecule. These reactions are carried out depending upon the amount of \[bromine\] and thus bromination takes place with or without the help of catalysts.
Complete answer:
\[\left( {3S} \right) - 3 - Methyl{\text{ }}Cyclohexene\] is an organic compound consisting \[Methyl\] as a functional group, an unsaturated carbon bond and a ring. The number on the structure represents the placement of these functional groups in the compound. The alphabet \[S\] represents the type of configuration. For that we must know the enantiomers. Enantiomers are non-imposable mirror images. The absolute configuration refers to the spatial arrangement of the atoms of a chiral molecular entity. The \[S\] and \[R\] configuration indicates the relative spatial orientation of each atom in a molecule with a non-superimposable mirror image. Here \[S\] means the spatial isomer that has its relative direction of priority order in an anticlockwise direction whereas \[R\] means in clockwise direction.
For that one must be familiar with the bromination process. As one can predict from the name that it involves the \[bromine\] element. Therefore bromination is the process or reaction in which \[bromine\] is introduced into a molecule and no other elements are introduced into a molecule. In a \[benzene\] ring bromination takes place by electrophilic aromatic substitution.
Now let's find out the reaction of \[\left( {3S} \right) - 3 - Methyl{\text{ }}Cyclohexene\] in \[1,2 - dichloroethane\] to find out the number of \[dibromo\] derivatives it produced.
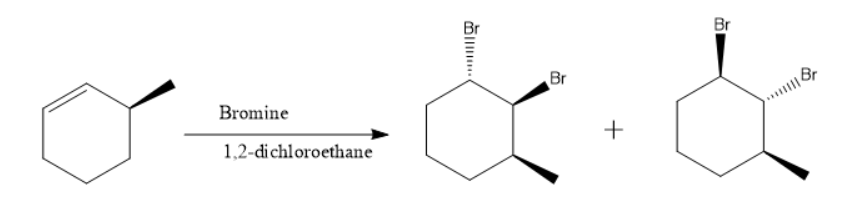
When \[bromine\] is added to \[\left( {3S} \right) - 3 - Methyl{\text{ }}Cyclohexene\] in \[1,2 - dichloroethane\] the number of \[dibromo\] derivatives formed are 2.
They are \[\left( {1S,2S,3S} \right) - 1,2 - dibromo - 3 - methylcyclohexane\] and \[\left( {1R,2R,3S} \right) - 1,2 - dibromo - 3 - methylcyclohexane\]
The reaction and their structure are as follow-
Note:
The products formed from the above reaction are similar, it is only the spatial orientation that differs. Also the knowledge of halogenation and electrophilic aromatic reaction will help you predict the product of the reaction of the reactants provided for such type of question.
Complete answer:
\[\left( {3S} \right) - 3 - Methyl{\text{ }}Cyclohexene\] is an organic compound consisting \[Methyl\] as a functional group, an unsaturated carbon bond and a ring. The number on the structure represents the placement of these functional groups in the compound. The alphabet \[S\] represents the type of configuration. For that we must know the enantiomers. Enantiomers are non-imposable mirror images. The absolute configuration refers to the spatial arrangement of the atoms of a chiral molecular entity. The \[S\] and \[R\] configuration indicates the relative spatial orientation of each atom in a molecule with a non-superimposable mirror image. Here \[S\] means the spatial isomer that has its relative direction of priority order in an anticlockwise direction whereas \[R\] means in clockwise direction.
For that one must be familiar with the bromination process. As one can predict from the name that it involves the \[bromine\] element. Therefore bromination is the process or reaction in which \[bromine\] is introduced into a molecule and no other elements are introduced into a molecule. In a \[benzene\] ring bromination takes place by electrophilic aromatic substitution.
Now let's find out the reaction of \[\left( {3S} \right) - 3 - Methyl{\text{ }}Cyclohexene\] in \[1,2 - dichloroethane\] to find out the number of \[dibromo\] derivatives it produced.
When \[bromine\] is added to \[\left( {3S} \right) - 3 - Methyl{\text{ }}Cyclohexene\] in \[1,2 - dichloroethane\] the number of \[dibromo\] derivatives formed are 2.
They are \[\left( {1S,2S,3S} \right) - 1,2 - dibromo - 3 - methylcyclohexane\] and \[\left( {1R,2R,3S} \right) - 1,2 - dibromo - 3 - methylcyclohexane\]
The reaction and their structure are as follow-

Note:
The products formed from the above reaction are similar, it is only the spatial orientation that differs. Also the knowledge of halogenation and electrophilic aromatic reaction will help you predict the product of the reaction of the reactants provided for such type of question.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




