
Number of possible geometrical isomers for $$2,4 - hexadiene$$ are?
(A) $8$
(B) $4$
(C) $3$
(D) $2$
Answer
561k+ views
Hint:Isomerism is a phenomenon of organic chemistry in which more than one species has the same chemical formula but different structures. Those compounds that have the same chemical formula but different properties and the arrangement of atoms in their molecule are called isomers.
Complete answer:
Isomerism is of two types (a) Structural and (b) Stereo. Geometrical isomerism is a type of stereoisomerism. Those molecules that have the same molecular formula but have different relative arrangements of atoms in space in their compounds are called geometrical isomers and this phenomenon is called geometrical isomerism.
This type of isomerism is shown by unsaturated compounds or by compounds that have a ring, in which rotation about the carbon bond is restricted which results in the formation of Cis and Trans configuration of the molecule. In the cis configuration, the functional group lies on the same side of the chain, and in the trans configuration, the functional group lies on the opposite side of the carbon chain.
In the question, we are given 2,4 - hexadiene. It is an alkene that is formed with a double bond ( = ) between the two carbon atoms. In the structure of 2,4 - hexadiene there are two double bonds between the carbon atoms at 2 and 4 position. So due to the presence of two double bonds, it will show three geometrical isomers as the two ends of the molecules are identically substituted. The three isomers will be:
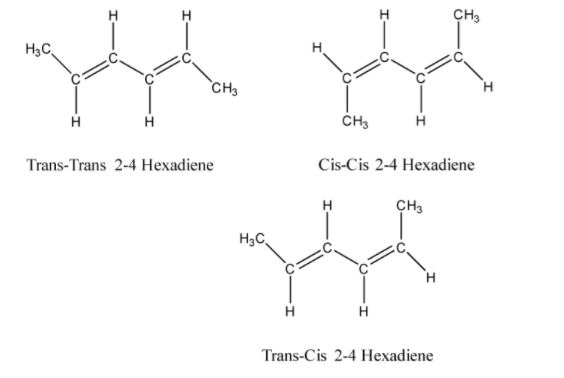
Hence, the Number of possible geometrical isomers for $$2,4 - hexadiene$$ are $3$.
Therefore, option (C) is correct.
Note:
The melting and boiling point of trans isomers is more than that of cis isomer. This is due to the reason that trans isomers are less polar and more symmetrical as compared to cis isomers who are more polar and less symmetrical.
Complete answer:
Isomerism is of two types (a) Structural and (b) Stereo. Geometrical isomerism is a type of stereoisomerism. Those molecules that have the same molecular formula but have different relative arrangements of atoms in space in their compounds are called geometrical isomers and this phenomenon is called geometrical isomerism.
This type of isomerism is shown by unsaturated compounds or by compounds that have a ring, in which rotation about the carbon bond is restricted which results in the formation of Cis and Trans configuration of the molecule. In the cis configuration, the functional group lies on the same side of the chain, and in the trans configuration, the functional group lies on the opposite side of the carbon chain.
In the question, we are given 2,4 - hexadiene. It is an alkene that is formed with a double bond ( = ) between the two carbon atoms. In the structure of 2,4 - hexadiene there are two double bonds between the carbon atoms at 2 and 4 position. So due to the presence of two double bonds, it will show three geometrical isomers as the two ends of the molecules are identically substituted. The three isomers will be:

Hence, the Number of possible geometrical isomers for $$2,4 - hexadiene$$ are $3$.
Therefore, option (C) is correct.
Note:
The melting and boiling point of trans isomers is more than that of cis isomer. This is due to the reason that trans isomers are less polar and more symmetrical as compared to cis isomers who are more polar and less symmetrical.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




