
Number of structural isomers possible in ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}$ are _______.
(A) 9
(B) 11
(C) 5
(D) 3
Answer
523.9k+ views
Hint: To find the number of structural isomers possible for a compound we must first know the molecular structure and the molecular formula of that compound as physical mind the number of carbon atoms present in the compound that can perform various linkage chains. I will provide all the possible isomers inclusive of the structures that are possible in ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}$ molecule.
Complete step by step solution:
> Structural isomers are the type of isomers in which the molecules with the same molecular formula consists of various bonding patterns and atomic organizations that are opposed to stereoisomers of molecular bond always in similar order and only spatial arrangements differ in them. In structural isomers, we can identify them with the bonding patterns. Usually they have the same atomic composition and the molecular number but they are connected in such ways that they differ in functional groups. Simple example of a structural isomer would be n-butane and isobutane where n-butane is a straight hydrocarbon chain with four carbon atoms while isobutane is branched in nature.
> The compound given in the question is ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}$ which has a common IUPAC name of propanal. The compound, as we can see, consists of 3 carbon atoms and 6 hydrogen atoms with the functional group of oxygen present in the formula. It is deficient in two hydrogen atoms relative to a saturated hydrocarbon. The structures present need a double bond to attain stability. The double bond can occur between two carbons or between oxygen and a carbon atom. This ring can be all carbon with an external hydronium ion oh. It can also include oxygen in the ring. The isomers of ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}$ include
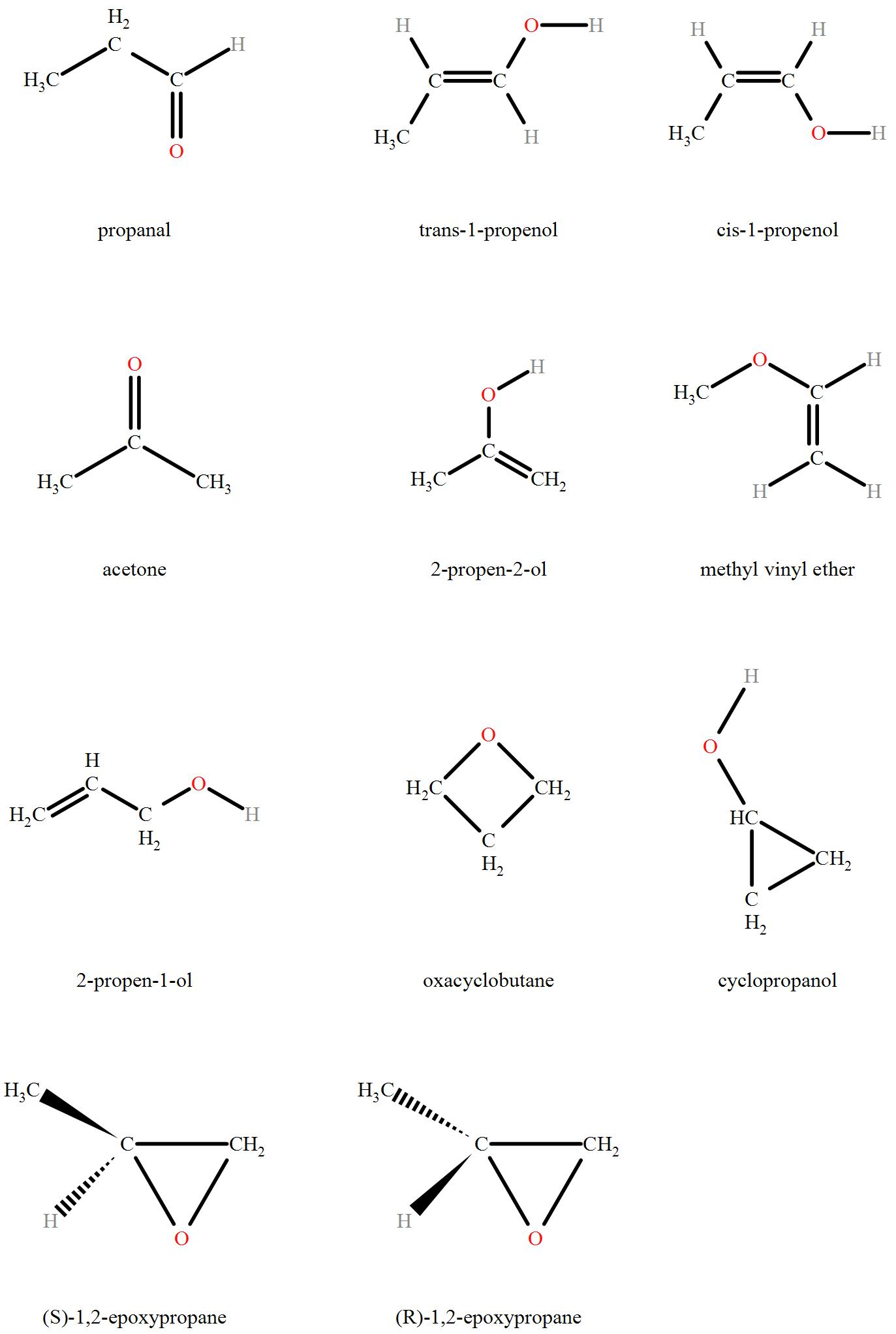
So as seen from the diagram, the 11 structural isomers are Propanal, Trans-1-propenol, cis-1-propenol, acetone, 2-propen-2-ol, methyl vinyl ether, 2-propen-1-ol, oxacyclobutane, cyclopropanol, (S)-1,2-epoxypropane, (R)-1,2-epoxypropane.
Hence we can see that a total of 11 structural isomers of ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}$ are possible.
Therefore, the correct option is Option B.
Note: In such cases, where we need to mention isomers, we always need to check the molecular formula of the given compound. With the molecular formula, we can form a molecular structure and depending on that structure, we can analyze if the compound is optically active, if it consists of structural or functional isomers, etc.
Complete step by step solution:
> Structural isomers are the type of isomers in which the molecules with the same molecular formula consists of various bonding patterns and atomic organizations that are opposed to stereoisomers of molecular bond always in similar order and only spatial arrangements differ in them. In structural isomers, we can identify them with the bonding patterns. Usually they have the same atomic composition and the molecular number but they are connected in such ways that they differ in functional groups. Simple example of a structural isomer would be n-butane and isobutane where n-butane is a straight hydrocarbon chain with four carbon atoms while isobutane is branched in nature.
> The compound given in the question is ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}$ which has a common IUPAC name of propanal. The compound, as we can see, consists of 3 carbon atoms and 6 hydrogen atoms with the functional group of oxygen present in the formula. It is deficient in two hydrogen atoms relative to a saturated hydrocarbon. The structures present need a double bond to attain stability. The double bond can occur between two carbons or between oxygen and a carbon atom. This ring can be all carbon with an external hydronium ion oh. It can also include oxygen in the ring. The isomers of ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}$ include

So as seen from the diagram, the 11 structural isomers are Propanal, Trans-1-propenol, cis-1-propenol, acetone, 2-propen-2-ol, methyl vinyl ether, 2-propen-1-ol, oxacyclobutane, cyclopropanol, (S)-1,2-epoxypropane, (R)-1,2-epoxypropane.
Hence we can see that a total of 11 structural isomers of ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}$ are possible.
Therefore, the correct option is Option B.
Note: In such cases, where we need to mention isomers, we always need to check the molecular formula of the given compound. With the molecular formula, we can form a molecular structure and depending on that structure, we can analyze if the compound is optically active, if it consists of structural or functional isomers, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




