
Nylon 6,6 is a ____.
A. Natural polymer
B. Synthetic polymer
C. Mixed polymer
D. Polyester
Answer
513.3k+ views
Hint: A substance consisting of very large molecules or consisting of long chain repeating units are known as polymers. The small units which form the polymers are known as monomers. The polymers are of two types i.e., natural polymer and synthetic polymer.
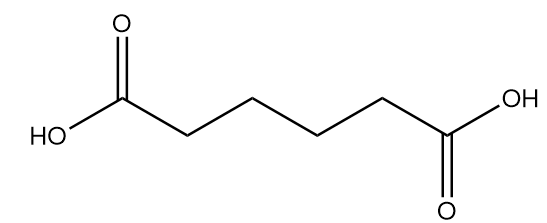
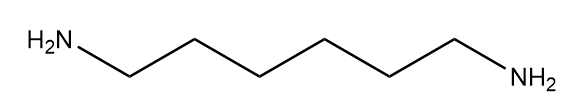
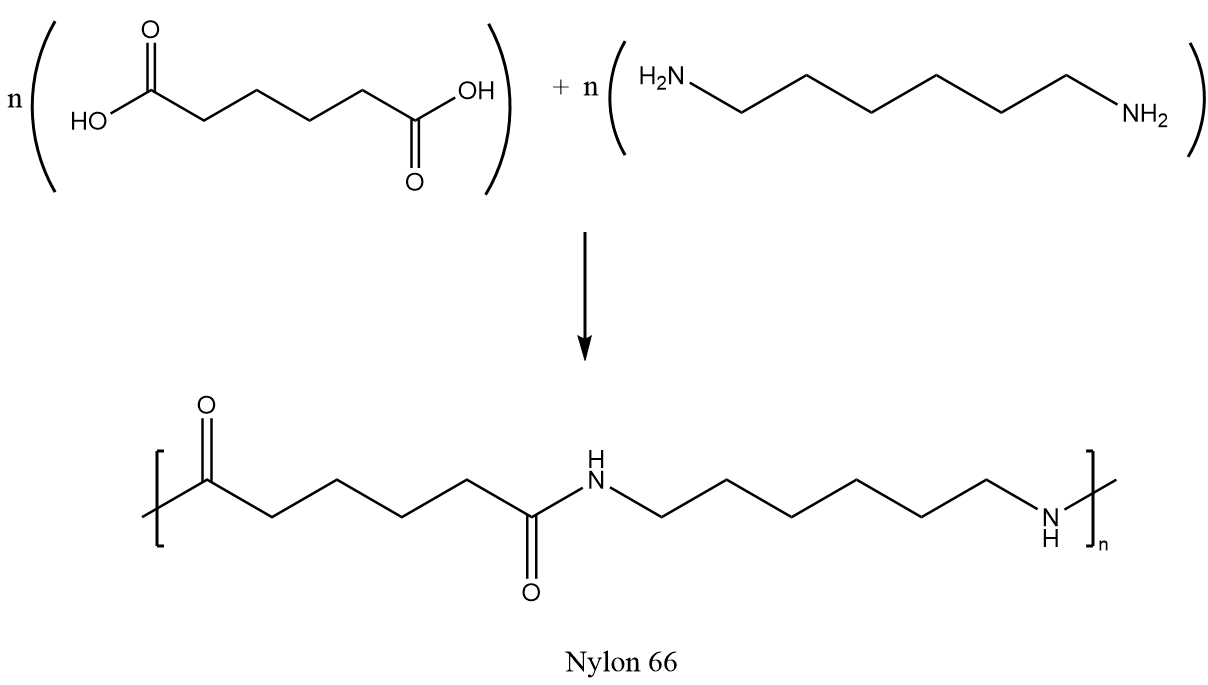
Complete answer: Nylon 6-6 is a synthetic polymer which is also called polyamide. The numbers describe the type and quantity of the polymer chain in the chemical structure. It is composed of two monomers which are hexamethylenediamine and adipic acid. The structures of the monomers of Nylon 66 are as follows:
Adipic acid:
Hexamethylenediamine:
Nylon 6,6 can be synthesized by a polycondensation reaction of adipic acid and hexamethylenediamine followed by the polymerization reaction. The synthesis reaction is as follows:
Hence, Nylon 6,6 is a synthetic polymer.
So, option (B) is the correct answer.
Additional information:
Some important properties of Nylon 6,6 are as follows:
-These polymers have high mechanical strength, hardness, stiffness and toughness.
-These polymers provide good resistance to high radiation energies like gamma and x ray.
-The water absorption rate for these polymers is comparatively lower than other polyamides.
-These polymers have good electrical insulating properties i.e., are good insulators of electricity.
Note:
It is important to note that the major difference between Nylon 6 and Nylon 6,6 polymers is mold shrinkage. The polymer nylon 6 has lower mold shrinkage which adds reliability to stuff whereas in nylon 6,6, due to its greater mold shrinkage, the shape of material or stuff changes by exposing it to cool air.
Complete answer: Nylon 6-6 is a synthetic polymer which is also called polyamide. The numbers describe the type and quantity of the polymer chain in the chemical structure. It is composed of two monomers which are hexamethylenediamine and adipic acid. The structures of the monomers of Nylon 66 are as follows:
Adipic acid:

Hexamethylenediamine:

Nylon 6,6 can be synthesized by a polycondensation reaction of adipic acid and hexamethylenediamine followed by the polymerization reaction. The synthesis reaction is as follows:

Hence, Nylon 6,6 is a synthetic polymer.
So, option (B) is the correct answer.
Additional information:
Some important properties of Nylon 6,6 are as follows:
-These polymers have high mechanical strength, hardness, stiffness and toughness.
-These polymers provide good resistance to high radiation energies like gamma and x ray.
-The water absorption rate for these polymers is comparatively lower than other polyamides.
-These polymers have good electrical insulating properties i.e., are good insulators of electricity.
Note:
It is important to note that the major difference between Nylon 6 and Nylon 6,6 polymers is mold shrinkage. The polymer nylon 6 has lower mold shrinkage which adds reliability to stuff whereas in nylon 6,6, due to its greater mold shrinkage, the shape of material or stuff changes by exposing it to cool air.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




