
o – Xylene on ozonolysis will give –
(A) Dimethyl glyoxal + Glyoxal
(B) Dimethyl glyoxal + Methylglyoxal
(C) Glyoxal + Methylglyoxal
(D) Dimethyl glyoxal + Methylglyoxal + Glyoxal
Answer
597.3k+ views
Hint: Ozonolysis gives a product in which the double bonds are replaced with an ozone molecule. Zn + ${{H}_{2}}O$ is a reducing agent. It causes the molecule to break off at the places where there is a carbon-oxygen-carbon bond. o – Xylene has 2 resonance structures. Hence, the products of ozonolysis of each structure will be different.
Complete answer:
- Ozonolysis is an organic reaction in which the oxidative cleavage of an unsaturated bond in a compound occurs when reacted with ozone. The end product will be an organic compound in which multiple carbon – carbon bonds have been replaced with bonds to oxygen.
- This product will then be treated with Zn + ${{H}_{2}}O$ which is a reducing agent. It causes the molecule to break off at the places where there is a carbon-oxygen-carbon bond.
- ortho Xylene actually has 2 resonance structures. One in which one of the pi bonds of benzene is in between the carbon atoms containing the alkyl groups, and the other in which there is no pi bond in between those carbon atoms.
- Hence ozonolysis can occur and lead to breaking off of bonds in 2 different ways as shown-
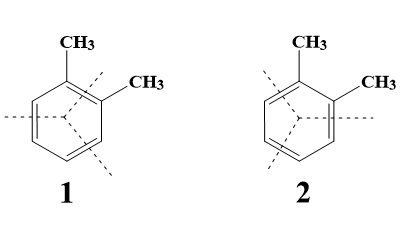
- Let us now see what will be the products of ozonolysis of each resonance structure of ortho Xylene.
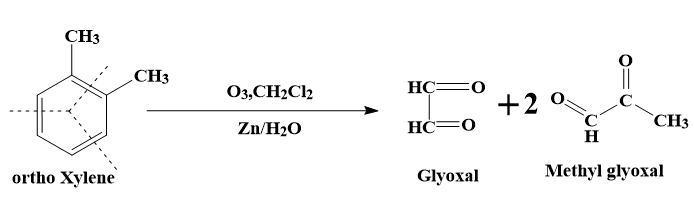
- The first resonance structure (1) will lead to the formation of 2 molecules of methyl glyoxal and one molecule of glyoxal.
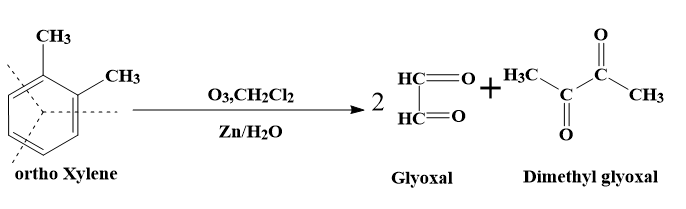
 - The second resonance structure (2) will lead to the formation of 2 molecules of glyoxal and one molecule of dimethyl glyoxal.
- The second resonance structure (2) will lead to the formation of 2 molecules of glyoxal and one molecule of dimethyl glyoxal.
 - Hence, we can conclude that the products of ozonolysis of ortho Xylene will be Dimethyl glyoxal, Methylglyoxal and Glyoxal.
- Hence, we can conclude that the products of ozonolysis of ortho Xylene will be Dimethyl glyoxal, Methylglyoxal and Glyoxal.
- Therefore, the answer will be option (D) Dimethyl glyoxal + Methylglyoxal + Glyoxal.
Note: It is important that you consider both the resonance structures of ortho Xylene while writing its products for ozonolysis reaction. If you consider only one of the resonance structures, you will get only 2 products. The answer will be incorrect as there are a total of 3 products in this reaction.
Complete answer:
- Ozonolysis is an organic reaction in which the oxidative cleavage of an unsaturated bond in a compound occurs when reacted with ozone. The end product will be an organic compound in which multiple carbon – carbon bonds have been replaced with bonds to oxygen.
- This product will then be treated with Zn + ${{H}_{2}}O$ which is a reducing agent. It causes the molecule to break off at the places where there is a carbon-oxygen-carbon bond.
- ortho Xylene actually has 2 resonance structures. One in which one of the pi bonds of benzene is in between the carbon atoms containing the alkyl groups, and the other in which there is no pi bond in between those carbon atoms.
- Hence ozonolysis can occur and lead to breaking off of bonds in 2 different ways as shown-

- Let us now see what will be the products of ozonolysis of each resonance structure of ortho Xylene.
- The first resonance structure (1) will lead to the formation of 2 molecules of methyl glyoxal and one molecule of glyoxal.


- Therefore, the answer will be option (D) Dimethyl glyoxal + Methylglyoxal + Glyoxal.
Note: It is important that you consider both the resonance structures of ortho Xylene while writing its products for ozonolysis reaction. If you consider only one of the resonance structures, you will get only 2 products. The answer will be incorrect as there are a total of 3 products in this reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




