
How will you obtain o-chlorotoluene from toluene?
Answer
460.5k+ views
Hint: Chlorotoluene exists in 3 isomeric forms- Ortho, Meta, and Para. This consists of a disubstituted benzene ring with one Chloro and one Methyl group. o-chlorotoluene or ortho chlorotoluene is actually a name given to 1-Chloro 2-Methyl toluene. It is a colorless liquid with a strong irritating odour.
Complete answer:
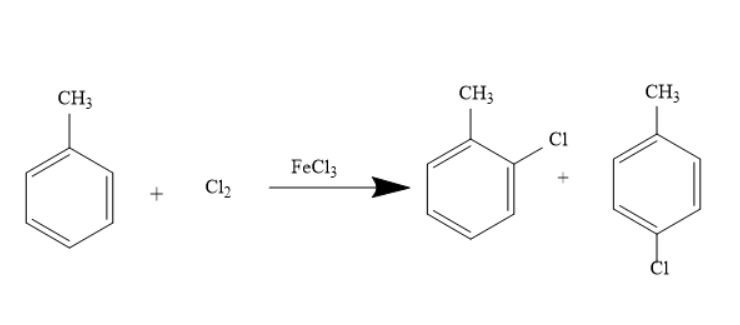
The preparation of o-chlorotoluene is possible from toluene in presence of Chlorine and Ferric chloride. This reaction is called the Chlorination reaction. It is a typical Electrophilic substitution reaction. Here Ferric chloride acts as the catalyst. The reaction is represented as under-
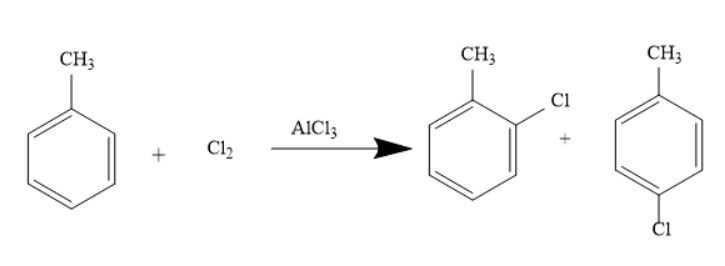
This reaction can also occur when toluene reacts in the presence of Chlorine and Aluminium Chloride. The reaction is represented as under-
The Chlorine is broken into $C{l^ - }$ and $C{l^ + }$ ions in the presence of a liquid catalyst. This $C{l^ + }$ ion (Electrophile) attacks the phenyl ring and hence results in the product. The Methyl group attached acts as an electron-donating group. The Methyl group in the toluene is ortho and para directing and thus the electrophilic substitution reaction occurs at the ortho and para position.
However, if the reaction occurs in absence of a catalyst the substitution occurs at the methyl group to form trichloromethyl benzene.
Note:
o-chlorotoluene has wide industrial applications. It is used as a bactericide and insecticide. It is also used in the preparation of dyes, Synthetic rubber, dyes, and pharmaceuticals. It is also used as a solvent and chemical intermediate. It can irritate the eye and skin when it comes in contact with it.
Complete answer:
The preparation of o-chlorotoluene is possible from toluene in presence of Chlorine and Ferric chloride. This reaction is called the Chlorination reaction. It is a typical Electrophilic substitution reaction. Here Ferric chloride acts as the catalyst. The reaction is represented as under-

This reaction can also occur when toluene reacts in the presence of Chlorine and Aluminium Chloride. The reaction is represented as under-

The Chlorine is broken into $C{l^ - }$ and $C{l^ + }$ ions in the presence of a liquid catalyst. This $C{l^ + }$ ion (Electrophile) attacks the phenyl ring and hence results in the product. The Methyl group attached acts as an electron-donating group. The Methyl group in the toluene is ortho and para directing and thus the electrophilic substitution reaction occurs at the ortho and para position.
However, if the reaction occurs in absence of a catalyst the substitution occurs at the methyl group to form trichloromethyl benzene.
Note:
o-chlorotoluene has wide industrial applications. It is used as a bactericide and insecticide. It is also used in the preparation of dyes, Synthetic rubber, dyes, and pharmaceuticals. It is also used as a solvent and chemical intermediate. It can irritate the eye and skin when it comes in contact with it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




