
O-cresol and benzyl alcohol are:
(A) Functional isomers
(B) Positional isomers
(C) Chain isomers
(D) All of the above
Answer
577.8k+ views
Hint: To solve this question first we have to draw the structure of the given compound i.e. o-cresol and benzyl alcohol. Isomers are the molecules having identical molecular formula but different arrangements of atoms.
Complete step by step solution:
Before solving this question let's understand the terms given in the options:
Functional isomers – these are the type of isomers which have the same molecular formula but the connectivity of atoms is different in ways and it falls into the different functional groups.
-Positional isomers – these are the types of isomers which have the same molecular formula but there is a difference in the position of the functional groups.
-Chain isomers – these are the types of isomers which have the same molecular formula but there is a difference in the arrangements of the carbon skeleton.
O-cresol and benzyl alcohol have the following structure:
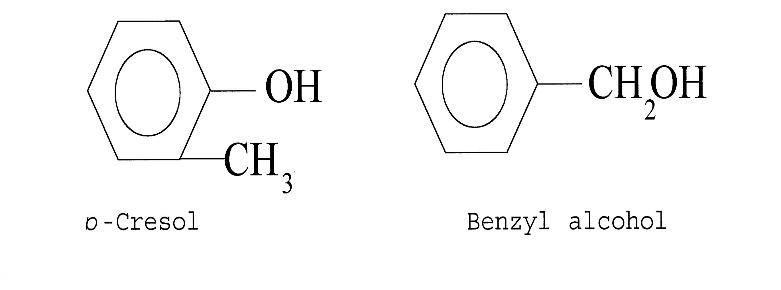
-They are functional isomers i.e. these are the type of isomers which have the same molecular formula but the connectivity of atoms is different in ways and it falls into the different functional groups.
Hence the correct answer is option (A) i.e. functional isomers
Additional information:
While deciding the kind of structural isomerism follow the following order to avoid the confusion:
Ring chain isomers
Tautomerism
Functional isomerism
Metamerism
Chain isomerism
Position isomerism
Note: Stereoisomers is another type of isomerism which is further divided into conformational and configurational isomerism. This kind of isomerism deals with the spatial arrangements or the orientation of the molecules atoms in a compound in space.
Complete step by step solution:
Before solving this question let's understand the terms given in the options:
Functional isomers – these are the type of isomers which have the same molecular formula but the connectivity of atoms is different in ways and it falls into the different functional groups.
-Positional isomers – these are the types of isomers which have the same molecular formula but there is a difference in the position of the functional groups.
-Chain isomers – these are the types of isomers which have the same molecular formula but there is a difference in the arrangements of the carbon skeleton.
O-cresol and benzyl alcohol have the following structure:
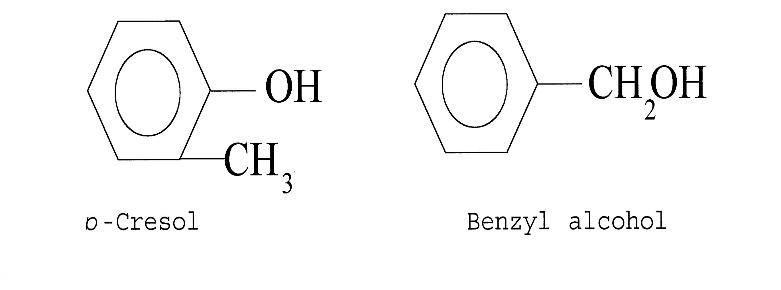
-They are functional isomers i.e. these are the type of isomers which have the same molecular formula but the connectivity of atoms is different in ways and it falls into the different functional groups.
Hence the correct answer is option (A) i.e. functional isomers
Additional information:
While deciding the kind of structural isomerism follow the following order to avoid the confusion:
Ring chain isomers
Tautomerism
Functional isomerism
Metamerism
Chain isomerism
Position isomerism
Note: Stereoisomers is another type of isomerism which is further divided into conformational and configurational isomerism. This kind of isomerism deals with the spatial arrangements or the orientation of the molecules atoms in a compound in space.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




