
On reacting with $ B{r_2} $ in $ CC{l_4}/C{H_2}C{l_2} $ , which among benzene and cyclohexane will react?
A. Benzene
B. Cyclohexane
C. Both
D. None
Answer
544.5k+ views
Hint :According to the question, first we will check, whether the benzene and cyclohexane will react separately with bromine or not. Then to know what happens after the reaction with $ B{r_2} $ in $ CC{l_4}/C{H_2}C{l_2} $ .
Complete Step By Step Answer:
Cyclohexane will take place free-radical reaction with bromine in the presence of light which is determined by the loss of orange colour of bromine upon the addition of cyclohexane and exposure of light for 10 minutes.
On the other hand, benzene cannot react with bromine upon exposure to the light since the bromination reaction of benzene will require a catalyst. The reaction given by
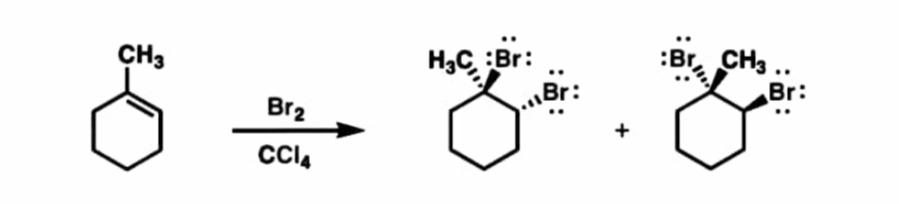
The orange colour of the test solution will be left upon addition of benzene and exposure to light for 10 minutes. Reactivity of Cyclohexene and Benzene However when bromine is added to benzene, the bromine remains orange and there is no reaction. This is because: Benzene has delocalised electrons spread over 6 carbon atoms, whereas alkenes have localised electrons above and below the 2 carbon atoms in the double bond.
Cyclohexane will not react with bromine unless the light or heat energy is applied. In such a case, a substitution free-radical reaction occurs. Cyclohexane is a kind of alkene, and benzene is aromatic compounds.
Hence, the correct option is (B.) Cyclohexane.
Note :
When cyclohexane reacts with bromine, a bromonium ion will form, and that ion will be attacked from the back by a bromide ion formed in a nearby reaction. The reactions of the cycloalkenes are generally the same as the cycloalkanes, with the exception of the very small ones- particularly cyclopropane.
Complete Step By Step Answer:
Cyclohexane will take place free-radical reaction with bromine in the presence of light which is determined by the loss of orange colour of bromine upon the addition of cyclohexane and exposure of light for 10 minutes.
On the other hand, benzene cannot react with bromine upon exposure to the light since the bromination reaction of benzene will require a catalyst. The reaction given by

The orange colour of the test solution will be left upon addition of benzene and exposure to light for 10 minutes. Reactivity of Cyclohexene and Benzene However when bromine is added to benzene, the bromine remains orange and there is no reaction. This is because: Benzene has delocalised electrons spread over 6 carbon atoms, whereas alkenes have localised electrons above and below the 2 carbon atoms in the double bond.
Cyclohexane will not react with bromine unless the light or heat energy is applied. In such a case, a substitution free-radical reaction occurs. Cyclohexane is a kind of alkene, and benzene is aromatic compounds.
Hence, the correct option is (B.) Cyclohexane.
Note :
When cyclohexane reacts with bromine, a bromonium ion will form, and that ion will be attacked from the back by a bromide ion formed in a nearby reaction. The reactions of the cycloalkenes are generally the same as the cycloalkanes, with the exception of the very small ones- particularly cyclopropane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




