
On reaction of chlorobenzene with acetyl chloride in presence of $AlC{{l}_{3}}$, the major product formed is-
(A) 1-Chloro-2-methylbenzene
(B) 1-Chloro-4-ethylbenzene
(C) 2-Chloro Acetophenone
(D) 4-Chloro Acetophenone
Answer
525.3k+ views
Hint: This is an example of a Friedel – Crafts acylation reaction. Cl atom is an ortho para directing group. Also take into consideration the steric effect and the inductive effect.
Complete answer:
The reaction of chlorobenzene with acetyl chloride in presence of $AlC{{l}_{3}}$ can also be called as Freidel – Crafts acylation of chlorobenzene.
Let us first understand the Friedel – Crafts reaction
The Friedel – Crafts reaction is called an organic coupling reaction.
This is because, in the Friedel – Crafts reaction, an organic aromatic substitution occurs which leads to attachment of a substituent to the aromatic ring.
For this reaction, $AlC{{l}_{3}}$ is used as a catalyst. The role of the catalyst is to extract chlorine atoms from the reactant and generate an electrophile.
Friedel – Crafts reaction is of 2 types - Friedel – Crafts alkylation and Friedel – Crafts acylation.
The reaction of chlorobenzene with acetyl chloride in presence of $AlC{{l}_{3}}$ is a Friedel – Crafts acylation reaction.
Friedel-Crafts acylation is a reaction in which the addition of an acyl group to an aromatic ring takes place. The reaction will lead to the aromatic ring being transformed into a ketone.
Our reactant is not benzene. It is chlorobenzene.
The Cl atom in Chlorobenzene is an ortho-para directing group. This is because Cl has a lone pair. Therefore, in the resonating structures of chlorobenzene, the electrons will be delocalised into the ring and the electron density on ortho and para position will be increased. The electron density will be more at ortho and para positions as there are resonating structures in which the negative charge is on the ortho para positions.
Hence, the Friedel – Crafts acylation of chlorobenzene will yield us both ortho and para products.
Therefore, the reaction of chlorobenzene with acetyl chloride in presence of $AlC{{l}_{3}}$, will yield the products - 2-Chloro Acetophenone and 4-Chloro Acetophenone.
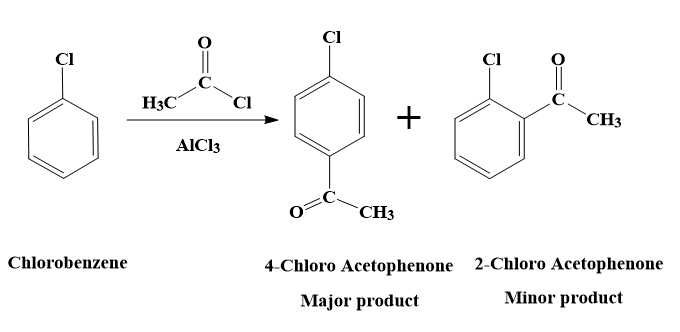 - Out of the 2 products, 4-Chloro Acetophenone will be the major product.
- Out of the 2 products, 4-Chloro Acetophenone will be the major product.
- This is because the steric hindrance at the ortho position and the -I effect of the Cl atom results in deactivation of the benzene ring.
Note: There are actually 2 products formed in the reaction. The major product will be decided by steric hindrance and inductive effect of the Cl atom. It is important to consider these effects while predicting the major product.
Complete answer:
The reaction of chlorobenzene with acetyl chloride in presence of $AlC{{l}_{3}}$ can also be called as Freidel – Crafts acylation of chlorobenzene.
Let us first understand the Friedel – Crafts reaction
The Friedel – Crafts reaction is called an organic coupling reaction.
This is because, in the Friedel – Crafts reaction, an organic aromatic substitution occurs which leads to attachment of a substituent to the aromatic ring.
For this reaction, $AlC{{l}_{3}}$ is used as a catalyst. The role of the catalyst is to extract chlorine atoms from the reactant and generate an electrophile.
Friedel – Crafts reaction is of 2 types - Friedel – Crafts alkylation and Friedel – Crafts acylation.
The reaction of chlorobenzene with acetyl chloride in presence of $AlC{{l}_{3}}$ is a Friedel – Crafts acylation reaction.
Friedel-Crafts acylation is a reaction in which the addition of an acyl group to an aromatic ring takes place. The reaction will lead to the aromatic ring being transformed into a ketone.
Our reactant is not benzene. It is chlorobenzene.
The Cl atom in Chlorobenzene is an ortho-para directing group. This is because Cl has a lone pair. Therefore, in the resonating structures of chlorobenzene, the electrons will be delocalised into the ring and the electron density on ortho and para position will be increased. The electron density will be more at ortho and para positions as there are resonating structures in which the negative charge is on the ortho para positions.
Hence, the Friedel – Crafts acylation of chlorobenzene will yield us both ortho and para products.
Therefore, the reaction of chlorobenzene with acetyl chloride in presence of $AlC{{l}_{3}}$, will yield the products - 2-Chloro Acetophenone and 4-Chloro Acetophenone.

- This is because the steric hindrance at the ortho position and the -I effect of the Cl atom results in deactivation of the benzene ring.
Note: There are actually 2 products formed in the reaction. The major product will be decided by steric hindrance and inductive effect of the Cl atom. It is important to consider these effects while predicting the major product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




