
When ortho dibromo benzene is subjected to mononitration X number of products are formed and when meta dibromo benzene is subjected to mononitration, Y number of products are formed. Report your answer as XY.
A.$31$
B.$22$
C.$23$
D.$32$
Answer
509.1k+ views
Hint: We know that carbon is a very versatile and important element. Its tetravalent nature allows it to bond with up to four elements at a time. Electron donating groups on the benzene ring directs the electrophiles to attack benzene at ortho and para positions.
Complete answer:
Carbon can form chains of compounds; this property is called catenation. The compound of carbon is tetravalent and thus can form many compounds of various properties. Carbon has a unique property of catenation, in that the carbon atom can form large chains of carbon atoms which are stable too. This property allows the carbon atom to form a chain and cyclic structures that exhibit large and varied properties.
One of the most popular examples of such property is benzene which is a six-membered ring. This compound also shows a property of aromaticity. It states that the electrons inside the benzene atom are not stationary but are continuously resonating. Resonance is the property which explains the property that the electrons in a compound are not stable by their stationary nature but are in continuous motion from one place to another within the atom.
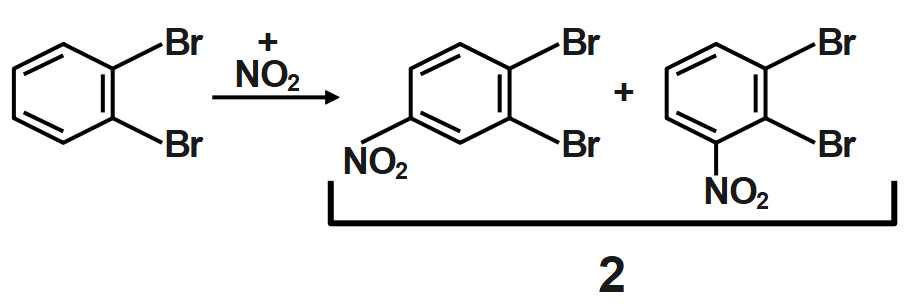
Because of the large number of electrons present surrounding the chlorine which is very nearer to ortho position it does not favor the formation of ortho-substituted products in major amounts. So, para-substituted product forms in a major amount. The electron-withdrawing effect of the chain is termed negative inductive effect and the electron-donating effect is termed as positive inductive effect.
Thus, ortho dibromo benzene is subjected to mononitration X number of products are formed and when meta dibromo benzene is subjected to mononitration, Y number of products are formed is $22.$
Therefore, the correct answer is option B.
Note:
Remember that the inductive effect is the effect due to the transmission of the inequality in the sharing of the electron during the bonding process through the chain of atoms in the molecule. It may be the reason for a permanent dipole in the compound due to this effect.
Complete answer:
Carbon can form chains of compounds; this property is called catenation. The compound of carbon is tetravalent and thus can form many compounds of various properties. Carbon has a unique property of catenation, in that the carbon atom can form large chains of carbon atoms which are stable too. This property allows the carbon atom to form a chain and cyclic structures that exhibit large and varied properties.
One of the most popular examples of such property is benzene which is a six-membered ring. This compound also shows a property of aromaticity. It states that the electrons inside the benzene atom are not stationary but are continuously resonating. Resonance is the property which explains the property that the electrons in a compound are not stable by their stationary nature but are in continuous motion from one place to another within the atom.

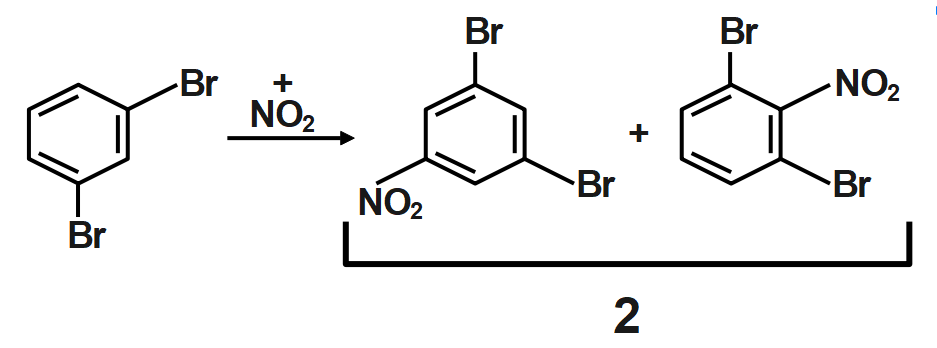
Because of the large number of electrons present surrounding the chlorine which is very nearer to ortho position it does not favor the formation of ortho-substituted products in major amounts. So, para-substituted product forms in a major amount. The electron-withdrawing effect of the chain is termed negative inductive effect and the electron-donating effect is termed as positive inductive effect.

Thus, ortho dibromo benzene is subjected to mononitration X number of products are formed and when meta dibromo benzene is subjected to mononitration, Y number of products are formed is $22.$
Therefore, the correct answer is option B.
Note:
Remember that the inductive effect is the effect due to the transmission of the inequality in the sharing of the electron during the bonding process through the chain of atoms in the molecule. It may be the reason for a permanent dipole in the compound due to this effect.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




