
Orthoboric acid ${{H}_{3}}B{{O}_{3}}$ and metaboric acid $HB{{O}_{2}}$ differ in respect of:
a.) Basicity
b.) Structure
c.) Melting point
d.) None of the above
Answer
567k+ views
Hint: To differentiate between orthoboric acid and metaboric acid on the basis of the given properties first we have to understand what these terms mean. The molecular formula of orthoboric acid is ${{H}_{3}}B{{O}_{3}}$ and metaboric acid is $HB{{O}_{2}}$.
Complete Solution :
In chemistry, a base is a substance which accepts hydrogen ions in water. Base neutralizes acid. Basicity is measured using a scale known as pH scale.
- Structure is the arrangement of atoms with respect to each other.
- Melting point is the temperature at which the given solid melts.
- If we talk about Orthoboric acid ${{H}_{3}}B{{O}_{3}}$ and metaboric acid $HB{{O}_{2}}$, the pKa value of orthoboric acid is 9.24, 12.4, 13.4 and the pKa value for the metaboric acid is 9.23 Hence, both of them have different acidity or basicity.
- The molecular formula of orthoboric acid is ${{H}_{3}}B{{O}_{3}}$ and metaboric acid is $HB{{O}_{2}}$. Hence, they differ in structure also. The structure of orthoboric acid and metaboric acid is mentioned below:
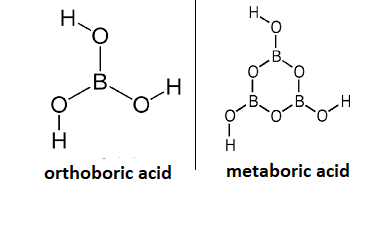
If we talk about the melting point of both the compounds. The melting point of orthoboric acid is 236 degree centigrade while that of metaboric acid is 170.09 degree Centigrade.
Hence, the correct answer is option (A), option (B), option (C) i.e. Orthoboric acid ${{H}_{3}}B{{O}_{3}}$ and metaboric acid $HB{{O}_{2}}$ differ in respect of basicity, structure and melting point. So, the correct answer is “OptionA, B and C”.
Note: It is very important to note that orthoboric acid is very poisonous if we inhale it in a very large quantity. A continued exposure to boric acid for a long period of time can also lead to kidney severely.
Complete Solution :
In chemistry, a base is a substance which accepts hydrogen ions in water. Base neutralizes acid. Basicity is measured using a scale known as pH scale.
- Structure is the arrangement of atoms with respect to each other.
- Melting point is the temperature at which the given solid melts.
- If we talk about Orthoboric acid ${{H}_{3}}B{{O}_{3}}$ and metaboric acid $HB{{O}_{2}}$, the pKa value of orthoboric acid is 9.24, 12.4, 13.4 and the pKa value for the metaboric acid is 9.23 Hence, both of them have different acidity or basicity.
- The molecular formula of orthoboric acid is ${{H}_{3}}B{{O}_{3}}$ and metaboric acid is $HB{{O}_{2}}$. Hence, they differ in structure also. The structure of orthoboric acid and metaboric acid is mentioned below:

If we talk about the melting point of both the compounds. The melting point of orthoboric acid is 236 degree centigrade while that of metaboric acid is 170.09 degree Centigrade.
Hence, the correct answer is option (A), option (B), option (C) i.e. Orthoboric acid ${{H}_{3}}B{{O}_{3}}$ and metaboric acid $HB{{O}_{2}}$ differ in respect of basicity, structure and melting point. So, the correct answer is “OptionA, B and C”.
Note: It is very important to note that orthoboric acid is very poisonous if we inhale it in a very large quantity. A continued exposure to boric acid for a long period of time can also lead to kidney severely.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




