
Osmosis is an example of active transport. True or False? Explain.
Answer
573.6k+ views
Hint: Osmosis is the spontaneous net migration of solvent molecules into a region of higher solvent concentration through a selectively permeable membrane in the direction that appears to equalise the concentrations of the solute on both sides.
Complete answer:
- Osmosis is a passive process. Hence, the given statement is False.
- Osmosis takes place from higher water potential to lower water potential.
- On dissolving solutes to water, the free energy of water (water potential) decreases.
- Osmosis operates on the basis of difference of water potential in between two solvents.The more solute dissolved in a solution, the less water potential it has.
- Passive transport doesn’t need any energy for its engagement where active transport mainly occurs against the concentration gradient with the help of energy.
- In biological systems, osmosis is a critical operation, since biological membranes are semi- permeable.
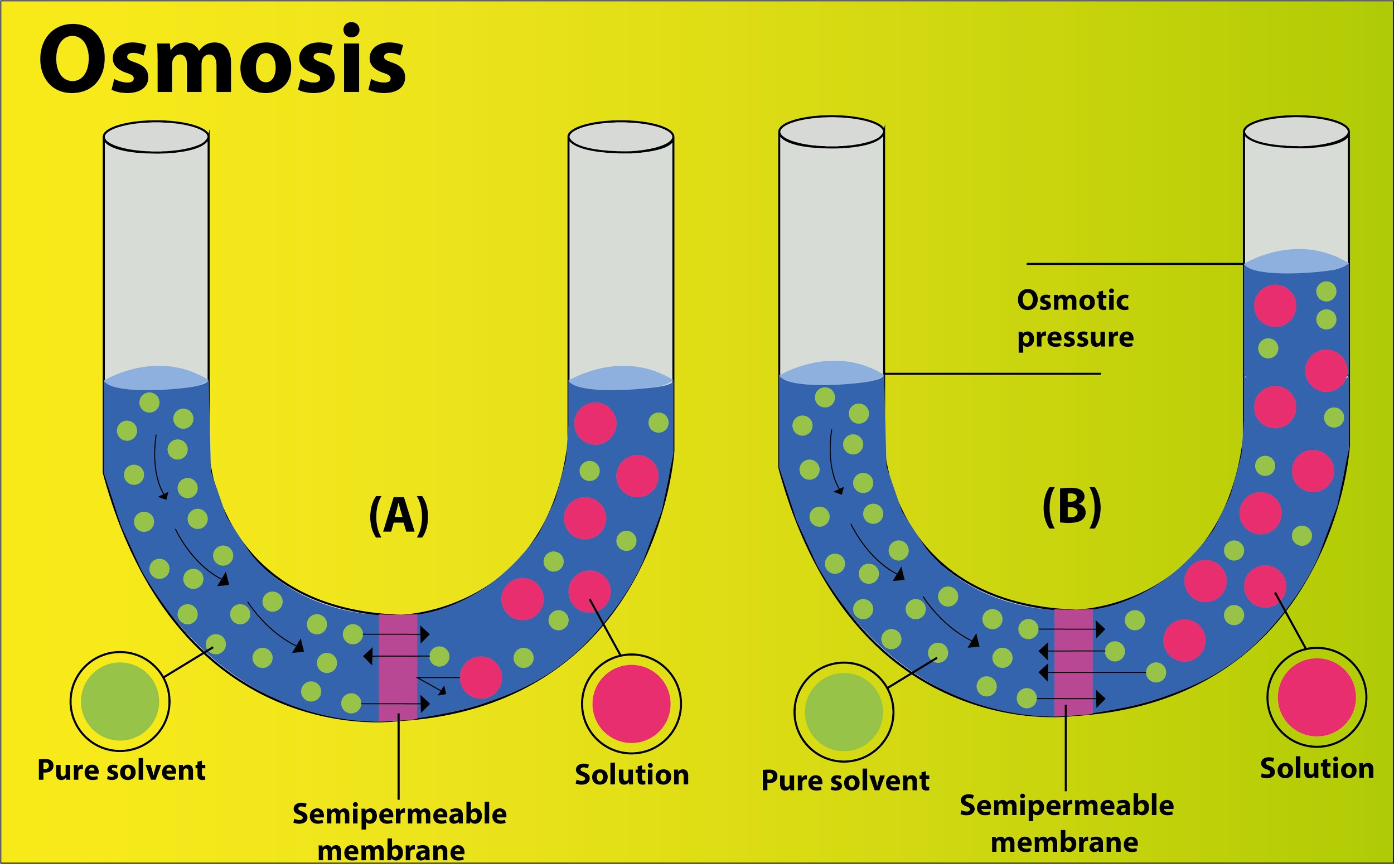
- These membranes are usually impermeable to large and polar molecules such as ions, proteins and polysaccharides, and are permeable to nonpolar or hydrophobic molecules such as lipids, as well as to small molecules such as oxygen , nitrogen, nitric oxide and carbon dioxide.
- By diffusing through the phospholipid bilayer through aquaporins (small transmembrane proteins similar to those responsible for facilitated diffusion and ion channels), water molecules travel through the plasma membrane, tonoplast (vacuole).
- Osmosis provides the main means of moving water into and out of cells.
- The cell turgor pressure is generally preserved between the cell interior and its comparatively hypotonic environments by osmosis across the cell membrane.
Note:
- The external pressure to be applied is known as osmotic pressure, so that there is no net movement of the solvent across the membrane.
- Osmotic pressure is a colligative property, which means that the osmotic pressure depends on the solution's molar concentration.
Complete answer:
- Osmosis is a passive process. Hence, the given statement is False.
- Osmosis takes place from higher water potential to lower water potential.
- On dissolving solutes to water, the free energy of water (water potential) decreases.
- Osmosis operates on the basis of difference of water potential in between two solvents.The more solute dissolved in a solution, the less water potential it has.
- Passive transport doesn’t need any energy for its engagement where active transport mainly occurs against the concentration gradient with the help of energy.
- In biological systems, osmosis is a critical operation, since biological membranes are semi- permeable.

- These membranes are usually impermeable to large and polar molecules such as ions, proteins and polysaccharides, and are permeable to nonpolar or hydrophobic molecules such as lipids, as well as to small molecules such as oxygen , nitrogen, nitric oxide and carbon dioxide.
- By diffusing through the phospholipid bilayer through aquaporins (small transmembrane proteins similar to those responsible for facilitated diffusion and ion channels), water molecules travel through the plasma membrane, tonoplast (vacuole).
- Osmosis provides the main means of moving water into and out of cells.
- The cell turgor pressure is generally preserved between the cell interior and its comparatively hypotonic environments by osmosis across the cell membrane.
Note:
- The external pressure to be applied is known as osmotic pressure, so that there is no net movement of the solvent across the membrane.
- Osmotic pressure is a colligative property, which means that the osmotic pressure depends on the solution's molar concentration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




