
What is the other name of the process in which alkaline hydrolysis of ester occurs?
A. dehydrogenation
B. dehydration
C. esterification
D. saponification
Answer
570k+ views
Hint: We know that an ester is a chemical compound formed when alcohol reacts with a carboxylic acid.
We also know that an alkaline solution is the solution of a base and water. During the alkaline hydrolysis, the nucleophilic substitution of $O{H^ - }$ occurs at $ - C = O$. This reaction produces carboxylic salt and water.
Complete answer:
We know that an ester is a chemical compound formed when alcohol reacts with a carboxylic acid. This process is called esterification. In this water molecule gets eliminated, therefore the type of reaction is a condensation reaction. It is a reversible process.
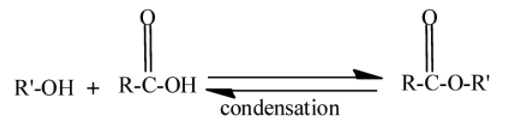
Here $ - R' - $ and $ - R - $ are alkyl groups. Hydrolysis is the process in which the water molecules are used to break the chemical compound. Alkaline solutions are the solutions of water and bases. Bases like $NaOH,KOH$ etc. Bases are the compounds which give $O{H^ - }$ in water. So when ester undergoes hydrolysis in an alkaline solution it gives carboxylic salt and water.
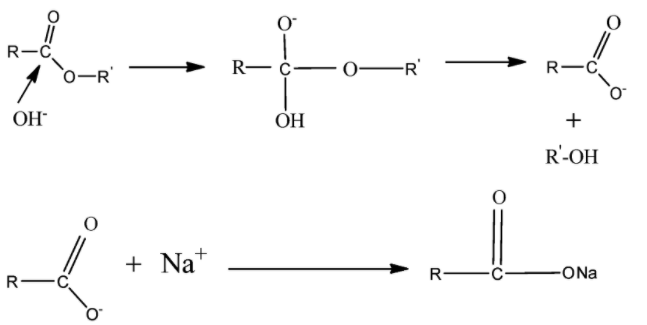
Here $NaOH$ breaks into $N{a^ + }$ and $O{H^ - }$ ions. $O{H^ - }$ acts as a nucleophile that is an electron-donating species and causes substitution reaction at $ - C = O$ . Alkaline hydrolysis of ester is also known as Saponification.
Therefore, the correct option is D. saponification
Note:
It is important to note that the hydrolysis of an ester is of two types. One is hydrolysis in an acidic medium and the other is hydrolysis in an alkaline medium. Acidic medium is the solution of an acid and water. The products in both mediums are different. In acidic hydrolysis, we get carboxylic acid and alcohol as products.
We also know that an alkaline solution is the solution of a base and water. During the alkaline hydrolysis, the nucleophilic substitution of $O{H^ - }$ occurs at $ - C = O$. This reaction produces carboxylic salt and water.
Complete answer:
We know that an ester is a chemical compound formed when alcohol reacts with a carboxylic acid. This process is called esterification. In this water molecule gets eliminated, therefore the type of reaction is a condensation reaction. It is a reversible process.

Here $ - R' - $ and $ - R - $ are alkyl groups. Hydrolysis is the process in which the water molecules are used to break the chemical compound. Alkaline solutions are the solutions of water and bases. Bases like $NaOH,KOH$ etc. Bases are the compounds which give $O{H^ - }$ in water. So when ester undergoes hydrolysis in an alkaline solution it gives carboxylic salt and water.

Here $NaOH$ breaks into $N{a^ + }$ and $O{H^ - }$ ions. $O{H^ - }$ acts as a nucleophile that is an electron-donating species and causes substitution reaction at $ - C = O$ . Alkaline hydrolysis of ester is also known as Saponification.
Therefore, the correct option is D. saponification
Note:
It is important to note that the hydrolysis of an ester is of two types. One is hydrolysis in an acidic medium and the other is hydrolysis in an alkaline medium. Acidic medium is the solution of an acid and water. The products in both mediums are different. In acidic hydrolysis, we get carboxylic acid and alcohol as products.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




